Phase 3 randomized trial on larynx preservation comparing sequential vs alternating chemotherapy and radiotherapy
- PMID: 19176454
- PMCID: PMC2724854
- DOI: 10.1093/jnci/djn460
Phase 3 randomized trial on larynx preservation comparing sequential vs alternating chemotherapy and radiotherapy
Abstract
Background: Both induction chemotherapy followed by irradiation and concurrent chemotherapy and radiotherapy have been reported as valuable alternatives to total laryngectomy in patients with advanced larynx or hypopharynx cancer. We report results of the randomized phase 3 trial 24954 from the European Organization for Research and Treatment of Cancer.
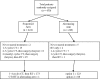
Methods: Patients with resectable advanced squamous cell carcinoma of the larynx (tumor stage T3-T4) or hypopharynx (T2-T4), with regional lymph nodes in the neck staged as N0-N2 and with no metastasis, were randomly assigned to treatment in the sequential (or control) or the alternating (or experimental) arm. In the sequential arm, patients with a 50% or more reduction in primary tumor size after two cycles of cisplatin and 5-fluorouracil received another two cycles, followed by radiotherapy (70 Gy total). In the alternating arm, a total of four cycles of cisplatin and 5-fluorouracil (in weeks 1, 4, 7, and 10) were alternated with radiotherapy with 20 Gy during the three 2-week intervals between chemotherapy cycles (60 Gy total). All nonresponders underwent salvage surgery and postoperative radiotherapy. The Kaplan-Meier method was used to obtain time-to-event data.
Results: The 450 patients were randomly assigned to treatment (224 to the sequential arm and 226 to the alternating arm). Median follow-up was 6.5 years. Survival with a functional larynx was similar in sequential and alternating arms (hazard ratio of death and/or event = 0.85, 95% confidence interval = 0.68 to 1.06), as were median overall survival (4.4 and 5.1 years, respectively) and median progression-free interval (3.0 and 3.1 years, respectively). Grade 3 or 4 mucositis occurred in 64 (32%) of the 200 patients in the sequential arm who received radiotherapy and in 47 (21%) of the 220 patients in the alternating arm. Late severe edema and/or fibrosis was observed in 32 (16%) patients in the sequential arm and in 25 (11%) in the alternating arm.
Conclusions: Larynx preservation, progression-free interval, and overall survival were similar in both arms, as were acute and late toxic effects.
Trial registration: ClinicalTrials.gov NCT00002839.
Figures

Comment in
-
Searching for less toxic larynx preservation: a need for common definitions and metrics.J Natl Cancer Inst. 2009 Feb 4;101(3):129-31. doi: 10.1093/jnci/djn490. Epub 2009 Jan 27. J Natl Cancer Inst. 2009. PMID: 19176460 No abstract available.
References
-
- Decker DA, Drelichman A, Jacob J, et al. Adjuvant chemotherapy with cisdiamminodichloroplatinum II and 120-hour infusion 5-fluorouracil in stage III and IV squamous cell carcinoma of the head and neck. Cancer. 1983;51(8):1353–1355. - PubMed
-
- Ensley J, Jacobs J, Weaver A, et al. Correlation between response to cisplatinum-combination chemotherapy and subsequent radiotherapy in previously untreated patients with advanced squamous cell cancers of the head and neck. Cancer. 1984;54(5):811–814. - PubMed
-
- Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med. 1991;324(24):1685–1690. - PubMed
-
- Lefebvre JL, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T. Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst. 1996;88(13):890–899. - PubMed
-
- Richard JM, Sancho-Garnier H, Pessey JJ, et al. Randomized trial of induction chemotherapy in larynx carcinoma. Oral Oncol. 1998;34(3):224–228. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

