Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice
- PMID: 19176467
- PMCID: PMC2662628
- DOI: 10.1210/er.2008-0025
Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice
Abstract
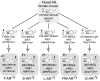
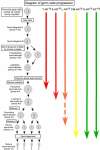
Androgens are critical steroid hormones that determine the expression of the male phenotype, including the outward development of secondary sex characteristics as well as the initiation and maintenance of spermatogenesis. Their actions are mediated by the androgen receptor (AR), a member of the nuclear receptor superfamily. AR functions as a ligand-dependent transcription factor, regulating expression of an array of androgen-responsive genes. Androgen and the AR play important roles in male spermatogenesis and fertility. The recent generation and characterization of male total and conditional AR knockout mice from different laboratories demonstrated the necessity of AR signaling for both external and internal male phenotype development. As expected, the male total AR knockout mice exhibited female-typical external appearance (including a vagina with a blind end and a clitoris-like phallus), the testis was located abdominally, and germ cell development was severely disrupted, which was similar to a human complete androgen insensitivity syndrome or testicular feminization mouse. However, the process of spermatogenesis is highly dependent on autocrine and paracrine communication among testicular cell types, and the disruption of AR throughout an experimental animal cannot answer the question about how AR in each type of testicular cell can play roles in the process of spermatogenesis. In this review, we provide new insights by comparing the results of cell-specific AR knockout in germ cells, peritubular myoid cells, Leydig cells, and Sertoli cells mouse models that were generated by different laboratories to see the consequent defects in spermatogenesis due to AR loss in different testicular cell types in spermatogenesis. Briefly, this review summarizes these results as follows: 1) the impact of lacking AR in Sertoli cells mainly affects Sertoli cell functions to support and nurture germ cells, leading to spermatogenesis arrest at the diplotene primary spermatocyte stage prior to the accomplishment of first meiotic division; 2) the impact of lacking AR in Leydig cells mainly affects steroidogenic functions leading to arrest of spermatogenesis at the round spermatid stage; 3) the impact of lacking AR in the smooth muscle cells and peritubular myoid cells in mice results in similar fertility despite decreased sperm output as compared to wild-type controls; and 4) the deletion of AR gene in mouse germ cells does not affect spermatogenesis and male fertility. This review tries to clarify the useful information regarding how androgen/AR functions in individual cells of the testis. The future studies of detailed molecular mechanisms in these in vivo animals with cell-specific AR knockout could possibly lead to useful insights for improvements in the treatment of male infertility, hypogonadism, and testicular dysgenesis syndrome, and in attempts to create safe as well as effective male contraceptive methods.
Figures


Similar articles
-
Oligozoospermia with normal fertility in male mice lacking the androgen receptor in testis peritubular myoid cells.Proc Natl Acad Sci U S A. 2006 Nov 21;103(47):17718-23. doi: 10.1073/pnas.0608556103. Epub 2006 Nov 9. Proc Natl Acad Sci U S A. 2006. PMID: 17095600 Free PMC article.
-
Differential effects of spermatogenesis and fertility in mice lacking androgen receptor in individual testis cells.Proc Natl Acad Sci U S A. 2006 Dec 12;103(50):18975-80. doi: 10.1073/pnas.0608565103. Epub 2006 Dec 1. Proc Natl Acad Sci U S A. 2006. PMID: 17142319 Free PMC article.
-
The androgen receptor in spermatogenesis.Cytogenet Genome Res. 2003;103(3-4):299-301. doi: 10.1159/000076816. Cytogenet Genome Res. 2003. PMID: 15051951 Review.
-
A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis.Proc Natl Acad Sci U S A. 2004 Feb 3;101(5):1327-32. doi: 10.1073/pnas.0308114100. Epub 2004 Jan 26. Proc Natl Acad Sci U S A. 2004. PMID: 14745012 Free PMC article.
-
Androgen receptor roles in spermatogenesis and infertility.Best Pract Res Clin Endocrinol Metab. 2015 Aug;29(4):595-605. doi: 10.1016/j.beem.2015.04.006. Epub 2015 Apr 25. Best Pract Res Clin Endocrinol Metab. 2015. PMID: 26303086 Review.
Cited by
-
Spermatogenesis arrest caused by conditional deletion of Hsp90α in adult mice.Biol Open. 2012 Oct 15;1(10):977-82. doi: 10.1242/bio.2012646. Epub 2012 Aug 17. Biol Open. 2012. PMID: 23213375 Free PMC article.
-
Improvements in in vitro spermatogenesis: oxygen concentration, antioxidants, tissue-form design, and space control.J Reprod Dev. 2024 Feb 19;70(1):1-9. doi: 10.1262/jrd.2023-093. Epub 2023 Dec 23. J Reprod Dev. 2024. PMID: 38143077 Free PMC article.
-
An Approach for Investigating Sexual Maturity in Wild Boar Males: Testosterone and 17β-Estradiol Analysis.Animals (Basel). 2022 Sep 5;12(17):2295. doi: 10.3390/ani12172295. Animals (Basel). 2022. PMID: 36078015 Free PMC article.
-
Kaliandra honey improves testosterone levels, diameter and epithelial thickness of seminiferous tubule of white rat (Rattus norvegicus) due to malnutrition through stimulation of HSP70.Open Vet J. 2021 Jul-Sep;11(3):401-406. doi: 10.5455/OVJ.2021.v11.i3.11. Epub 2021 Aug 10. Open Vet J. 2021. PMID: 34722203 Free PMC article.
-
The Histochemistry and Cell Biology omnium-gatherum: the year 2015 in review.Histochem Cell Biol. 2016 Mar;145(3):239-74. doi: 10.1007/s00418-016-1417-8. Epub 2016 Feb 15. Histochem Cell Biol. 2016. PMID: 26878854 Review.
References
-
- Chang JA, Nguyen HT, Lue TF 2002 Androgens in penile development, penile erection, and erectile dysfunction. In: Chang C, ed. Androgens and androgen receptor: mechanisms, functions and clinical applications. Norwell, MA: Kluwer Academic Publishers; 289–298
-
- Collins LL, Chang C 2002 Androgens and the androgen receptor in male sex development and fertility. In: Chang C, ed. Androgens and androgen receptor: mechanisms, functions and clinical applications. Norwell, MA: Kluwer Academic Publishers; 299–323
-
- Shumazaki S 2002 The role of 5-α reductase in prostate disease and male pattern baldness. In: Chang C, ed. Androgens and androgen receptor: mechanisms, functions and clinical applications. Norwell, MA: Kluwer Academic Publishers; 155–196
-
- Quigley CA 1998 The androgen receptor: physiology and pathophysiology. In: Nieschlag E, Behre HM, eds. Testosterone: action, deficiency, substitution. Heidelberg: Springer-Verlag; 33–106
-
- Chang CS, Kokontis J, Liao ST 1988 Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science 240:324–326 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

