Neurovascular development uses VEGF-A signaling to regulate blood vessel ingression into the neural tube
- PMID: 19176586
- PMCID: PMC2685948
- DOI: 10.1242/dev.028845
Neurovascular development uses VEGF-A signaling to regulate blood vessel ingression into the neural tube
Abstract
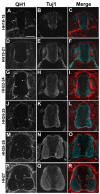
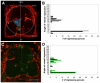
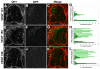
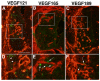
Neurovascular development requires communication between two developing organs, the neuroepithelium and embryonic blood vessels. We investigated the role of VEGF-A signaling in the embryonic crosstalk required for ingression of angiogenic vessel sprouts into the developing neural tube. As the neural tube develops, blood vessels enter at specific points medially and ventrally from the surrounding perineural vascular plexus. Localized ectopic expression of heparin-binding VEGF165 or VEGF189 from the developing avian neural tube resulted in supernumerary blood vessel ingression points and disrupted vessel patterning. By contrast, localized ectopic neural expression of non-heparin-binding VEGF121 did not produce supernumerary blood vessel ingression points, although the vessels that entered the neural tube became dysmorphogenic. Localized loss of endogenous VEGF-A signaling in the developing neural tube via ectopic expression of the VEGF inhibitor sFlt-1 locally blocked blood vessel ingression. The VEGF pathway manipulations were temporally controlled and did not dramatically affect neural tube maturation and dorsal-ventral patterning. Thus, neural-derived VEGF-A has a direct role in the spatially localized molecular crosstalk that is required for neurovascular development and vessel patterning in the developing neural tube.
Figures

References
-
- Aitkenhead, M., Christ, B., Eichmann, A., Feucht, M., Wilson, D. J. and Wilting, J. (1998). Paracrine and autocrine regulation of vascular endothelial growth factor during tissue differentiation in the quail. Dev. Dyn. 212, 1-13. - PubMed
-
- Ambler, C. A., Nowicki, J. L., Burke, A. C. and Bautch, V. L. (2001). Assembly of trunk and limb blood vessels involves extensive migration and vasculogenesis of somite-derived angioblasts. Dev. Biol. 234, 352-364. - PubMed
-
- Ambler, C. A., Schmunk, G. A. and Bautch, V. L. (2003). Stem cell-derived endothelial cells/progenitors migrate and pattern in the embryo using the VEGF signaling pathway. Dev. Biol. 257, 205-219. - PubMed
-
- Carmeliet, P. and Tessier-Lavigne, M. (2005). Common mechanisms of nerve and blood vessel wiring. Nature 436, 193-200. - PubMed
-
- Carmeliet, P., Ferreira, V., Breier, G., Pollefeyt, S., Kieckens, L., Gertsenstein, M., Fahrig, M., Vandenhoeck, A., Harpal, K., Eberhardt, C. et al. (1996). Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380, 435-439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

