Coordinate integrin and c-Met signaling regulate Wnt gene expression during epithelial morphogenesis
- PMID: 19176588
- PMCID: PMC2685949
- DOI: 10.1242/dev.027805
Coordinate integrin and c-Met signaling regulate Wnt gene expression during epithelial morphogenesis
Abstract
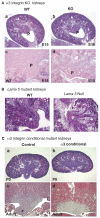
Integrin receptors for the extracellular matrix and receptor tyrosine kinase growth factor receptors represent two of the major families of receptors that transduce into cells information about the surrounding environment. Wnt proteins are a major family of signaling molecules that regulate morphogenetic events. There is presently little understanding of how the expression of Wnt genes themselves is regulated. In this study, we demonstrate that alpha3beta1 integrin, a major laminin receptor involved in the development of the kidney, and c-Met, the receptor for hepatocyte growth factor, signal coordinately to regulate the expression of Wnt7b in the mouse. Wnt signals in turn appear to regulate epithelial cell survival in the papilla of the developing kidney, allowing for the elongation of epithelial tubules to form a mature papilla. Together, these results demonstrate how signals from integrins and growth factor receptors can be integrated to regulate the expression of an important family of signaling molecules so as to regulate morphogenetic events.
Figures






References
-
- Anton, E. S., Kreidberg, J. A. and Rakic, P. (1999). Distinct functions of alpha3 and alpha(v) integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron 22, 277-289. - PubMed
-
- Birchmeier, C. and Gherardi, E. (1998). Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 8, 404-410. - PubMed
-
- Carroll, T. J., Park, J. S., Hayashi, S., Majumdar, A. and McMahon, A. P. (2005). Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev. Cell 9, 283-292. - PubMed
-
- Cebrian, C., Borodo, K., Charles, N. and Herzlinger, D. A. (2004). Morphometric index of the developing murine kidney. Dev. Dyn. 231, 601-608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

