Cortically activated interneurons shape spatial aspects of cortico-accumbens processing
- PMID: 19176610
- PMCID: PMC2695640
- DOI: 10.1152/jn.91002.2008
Cortically activated interneurons shape spatial aspects of cortico-accumbens processing
Abstract
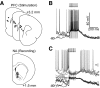
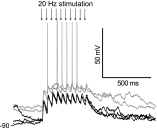
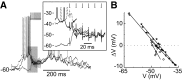
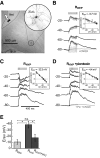
Basal ganglia circuits are organized as parallel loops that have been proposed to compete in a winner-take-all fashion to determine the appropriate behavioral outcome. However, limited experimental support for strong lateral inhibition mechanisms within striatal regions questions this model. Here, stimulation of the prefrontal cortex (PFC) using naturally occurring bursty patterns inhibited firing in most nucleus accumbens (NA) projection neurons. When an excitatory response was observed for one stimulation site, neighboring PFC sites evoked inhibition in the same neuron. Furthermore, PFC stimulation activated interneurons, and PFC-evoked inhibition was blocked by GABA(A) antagonists in corticoaccumbens slice preparations. Thus bursting PFC activity recruits local inhibition in the NA, shaping responses of projection neurons with a topographical arrangement that allows inhibition among parallel corticoaccumbens channels. The data indicate a high order of information processing within striatal circuits that should be considered in models of basal ganglia function and disease.
Figures


References
-
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res 85: 119–146, 1990. - PubMed
-
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8: 45–56, 2007. - PubMed
-
- Bennett BD, Bolam JP. Synaptic input and output of parvalbumin-immunoreactive neurons in the neostriatum of the rat. Neuroscience 62: 707–719, 1994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

