Polyamines regulate E-cadherin transcription through c-Myc modulating intestinal epithelial barrier function
- PMID: 19176757
- PMCID: PMC2670658
- DOI: 10.1152/ajpcell.00620.2008
Polyamines regulate E-cadherin transcription through c-Myc modulating intestinal epithelial barrier function
Abstract
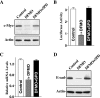
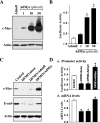
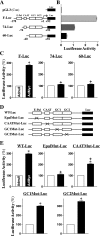
The integrity of the intestinal epithelial barrier depends on intercellular junctions that are highly regulated by numerous extracellular and intracellular factors. E-cadherin is found primarily at the adherens junctions in the intestinal mucosa and mediates strong cell-cell contacts that have a functional role in forming and regulating the epithelial barrier. Polyamines are necessary for E-cadherin expression, but the exact mechanism underlying polyamines remains elusive. The current study was performed to determine whether polyamines induce E-cadherin expression through the transcription factor c-Myc and whether polyamine-regulated E-cadherin plays a role in maintenance of the epithelial barrier integrity. Decreasing cellular polyamines reduced c-Myc and repressed E-cadherin transcription as indicated by a decrease in levels of E-cadherin promoter activity and its mRNA. Forced expression of the c-myc gene by infection with adenoviral vector containing c-Myc cDNA stimulated E-cadherin promoter activity and increased E-cadherin mRNA and protein levels in polyamine-deficient cells. Experiments using different E-cadherin promoter mutants revealed that induction of E-cadherin transcription by c-Myc was mediated through the E-Pal box located at the proximal region of the E-cadherin promoter. Decreased levels of E-cadherin in polyamine-deficient cells marginally increased basal levels of paracellular permeability but, remarkably, potentiated H(2)O(2)-induced epithelial barrier dysfunction. E-cadherin silencing by transfection with its specific small interfering RNA also increased vulnerability of the epithelial barrier to H(2)O(2). These results indicate that polyamines enhance E-cadherin transcription by activating c-Myc, thus promoting function of the epithelial barrier.
Figures

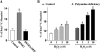
Similar articles
-
Polyamine-modulated c-Myc expression in normal intestinal epithelial cells regulates p21Cip1 transcription through a proximal promoter region.Biochem J. 2006 Sep 1;398(2):257-67. doi: 10.1042/BJ20060217. Biochem J. 2006. PMID: 16706751 Free PMC article.
-
p53-dependent NDRG1 expression induces inhibition of intestinal epithelial cell proliferation but not apoptosis after polyamine depletion.Am J Physiol Cell Physiol. 2007 Jul;293(1):C379-89. doi: 10.1152/ajpcell.00547.2006. Epub 2007 Apr 18. Am J Physiol Cell Physiol. 2007. PMID: 17442733
-
Polyamines are required for expression of Toll-like receptor 2 modulating intestinal epithelial barrier integrity.Am J Physiol Gastrointest Liver Physiol. 2007 Sep;293(3):G568-76. doi: 10.1152/ajpgi.00201.2007. Epub 2007 Jun 28. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17600044
-
Polyamines regulate expression of E-cadherin and play an important role in control of intestinal epithelial barrier function.Inflammopharmacology. 2005;13(1-3):91-101. doi: 10.1163/156856005774423890. Inflammopharmacology. 2005. PMID: 16259731 Review.
-
Polyamines in Gut Epithelial Renewal and Barrier Function.Physiology (Bethesda). 2020 Sep 1;35(5):328-337. doi: 10.1152/physiol.00011.2020. Physiology (Bethesda). 2020. PMID: 32783609 Free PMC article. Review.
Cited by
-
c-Jun enhances intestinal epithelial restitution after wounding by increasing phospholipase C-γ1 transcription.Am J Physiol Cell Physiol. 2017 Apr 1;312(4):C367-C375. doi: 10.1152/ajpcell.00330.2016. Epub 2017 Jan 18. Am J Physiol Cell Physiol. 2017. PMID: 28100486 Free PMC article.
-
Post-transcriptional regulation of MEK-1 by polyamines through the RNA-binding protein HuR modulating intestinal epithelial apoptosis.Biochem J. 2010 Feb 24;426(3):293-306. doi: 10.1042/BJ20091459. Biochem J. 2010. PMID: 20001965 Free PMC article.
-
Involvement of Antizyme Characterized from the Small Abalone Haliotis diversicolor in Gonadal Development.PLoS One. 2015 Aug 27;10(8):e0135251. doi: 10.1371/journal.pone.0135251. eCollection 2015. PLoS One. 2015. PMID: 26313647 Free PMC article.
-
Anti-inflammatory Gut Microbial Pathways Are Decreased During Crohn's Disease Exacerbations.J Crohns Colitis. 2019 Oct 28;13(11):1439-1449. doi: 10.1093/ecco-jcc/jjz077. J Crohns Colitis. 2019. PMID: 31066440 Free PMC article.
-
MicroRNA-195 regulates Tuft cell function in the intestinal epithelium by altering translation of DCLK1.Am J Physiol Cell Physiol. 2021 Jun 1;320(6):C1042-C1054. doi: 10.1152/ajpcell.00597.2020. Epub 2021 Mar 31. Am J Physiol Cell Physiol. 2021. PMID: 33788631 Free PMC article.
References
-
- Angst BD, Marcozzi C, Magee AI. The cadherin superfamily. J Cell Sci 114: 625–626, 2001. - PubMed
-
- Angst BD, Marcozzi C, Magee AI. The cadherin superfamily: diversity in form and function. J Cell Sci 114: 629–641, 2001. - PubMed
-
- Balda MS, Whitney JA, Flores C, González S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol 134: 1031–1049, 1996. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

