Ferumoxytol as an intravenous iron replacement therapy in hemodialysis patients
- PMID: 19176796
- PMCID: PMC2637590
- DOI: 10.2215/CJN.02840608
Ferumoxytol as an intravenous iron replacement therapy in hemodialysis patients
Abstract
Background and objectives: Intravenous iron is a key component of anemia management for chronic kidney disease (CKD). Ferumoxytol is a unique intravenous iron product that can be administered as a rapid injection in doses up to 510 mg.
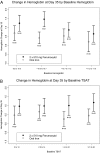
Design, setting, participants, & measurements: This was a randomized, open-label, controlled, multicenter Phase 3 trial to evaluate the safety and efficacy of intravenous ferumoxytol compared with oral iron. Anemic patients with CKD stage 5D on hemodialysis and on a stable erythropoiesis-stimulating agent regimen received either two injections of 510 mg of ferumoxytol within 7 d (n = 114) or 200 mg elemental oral iron daily for 21 d (n = 116). The primary efficacy endpoint was the change in hemoglobin from baseline to day 35. Safety was closely monitored.
Results: Ferumoxytol resulted in a mean increase in hemoglobin of 1.02 +/- 1.13 g/dl at day 35 compared with 0.46 +/- 1.06 g/dl with oral iron (P = 0.0002). Twice as many ferumoxytol-treated patients than oral iron-treated patients achieved a > or =1 g/dl hemoglobin increase at day 35 (P = 0.0002). There was a greater mean increase in transferrin saturation (TSAT) with ferumoxytol compared with oral iron at day 35 (P < 0.0001). The larger hemoglobin increase after ferumoxytol compared with oral iron at day 35 persisted after adjustment for baseline hemoglobin, TSAT, and serum ferritin. Overall adverse event rates were comparable between groups.
Conclusions: In patients on hemodialysis, rapid intravenous injection of 510 mg of ferumoxytol led to significantly greater hemoglobin increases compared with oral iron, with comparable tolerability.
Trial registration: ClinicalTrials.gov NCT00233597.
Figures


References
-
- Obrador GT, Ruthazer R, Arora P, Kausz AT, Pereira BJ: Prevalence of and factors associated with suboptimal care before initiation of dialysis in the United States. J Am Soc Nephrol 10: 1793–1800, 1999 - PubMed
-
- Astor BC, Muntner P, Levin A, Eustace JA, Coresh J: Association of kidney function with anemia: The Third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med 162: 1401–1408, 2002 - PubMed
-
- Hsu CY, McCulloch CE, Curhan GC: Iron status and hemoglobin level in chronic renal insufficiency. J Am Soc Nephrol 13: 2783–2786, 2002 - PubMed
-
- Gotloib L, Silverberg D, Fudin R, Shostak A: Iron deficiency is a common cause of anemia in chronic kidney disease and can often be corrected with intravenous iron. J Nephrol 19: 161–167, 2006 - PubMed
-
- Fishbane S, Maesaka JK: Iron management in end-stage renal disease. Am J Kidney Dis 29: 319–333, 1997 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

