Synaptopodin regulates plasticity of dendritic spines in hippocampal neurons
- PMID: 19176811
- PMCID: PMC6665122
- DOI: 10.1523/JNEUROSCI.5528-08.2009
Synaptopodin regulates plasticity of dendritic spines in hippocampal neurons
Abstract
The spine apparatus is an essential component of dendritic spines of cortical and hippocampal neurons, yet its functions are still enigmatic. Synaptopodin (SP), an actin-binding protein, is tightly associated with the spine apparatus and it may play a role in synaptic plasticity, but it has not yet been linked mechanistically to synaptic functions. We studied endogenous and transfected SP in dendritic spines of cultured hippocampal neurons and found that spines containing SP generate larger responses to flash photolysis of caged glutamate than SP-negative ones. An NMDA-receptor-mediated chemical long-term potentiation caused the accumulation of GFP-GluR1 in spine heads of control but not of shRNA-transfected, SP-deficient neurons. SP is linked to calcium stores, because their pharmacological blockade eliminated SP-related enhancement of glutamate responses, and release of calcium from stores produced an SP-dependent increase of GluR1 in spines. Thus, SP plays a crucial role in the calcium store-associated ability of neurons to undergo long-term plasticity.
Figures


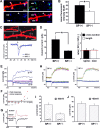

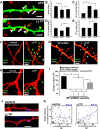
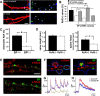



Similar articles
-
The spine apparatus, synaptopodin, and dendritic spine plasticity.Neuroscientist. 2010 Apr;16(2):125-31. doi: 10.1177/1073858409355829. Neuroscientist. 2010. PMID: 20400711 Review.
-
Synaptopodin regulates spine plasticity: mediation by calcium stores.J Neurosci. 2014 Aug 27;34(35):11641-51. doi: 10.1523/JNEUROSCI.0381-14.2014. J Neurosci. 2014. PMID: 25164660 Free PMC article.
-
Synaptopodin regulates release of calcium from stores in dendritic spines of cultured hippocampal neurons.J Physiol. 2011 Dec 15;589(Pt 24):5987-95. doi: 10.1113/jphysiol.2011.217315. Epub 2011 Oct 24. J Physiol. 2011. PMID: 22025667 Free PMC article.
-
Synaptopodin regulates denervation-induced homeostatic synaptic plasticity.Proc Natl Acad Sci U S A. 2013 May 14;110(20):8242-7. doi: 10.1073/pnas.1213677110. Epub 2013 Apr 29. Proc Natl Acad Sci U S A. 2013. PMID: 23630268 Free PMC article.
-
Understanding the role of synaptopodin and the spine apparatus in Hebbian synaptic plasticity - New perspectives and the need for computational modeling.Neurobiol Learn Mem. 2017 Feb;138:21-30. doi: 10.1016/j.nlm.2016.07.023. Epub 2016 Jul 25. Neurobiol Learn Mem. 2017. PMID: 27470091 Review.
Cited by
-
Repetitive magnetic stimulation induces functional and structural plasticity of excitatory postsynapses in mouse organotypic hippocampal slice cultures.J Neurosci. 2012 Nov 28;32(48):17514-23. doi: 10.1523/JNEUROSCI.0409-12.2012. J Neurosci. 2012. PMID: 23197741 Free PMC article.
-
Stim and Orai proteins in neuronal Ca(2+) signaling and excitability.Front Cell Neurosci. 2015 Apr 24;9:153. doi: 10.3389/fncel.2015.00153. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25964739 Free PMC article. Review.
-
Myosin V regulates synaptopodin clustering and localization in the dendrites of hippocampal neurons.J Cell Sci. 2019 Aug 22;132(16):jcs230177. doi: 10.1242/jcs.230177. J Cell Sci. 2019. PMID: 31371487 Free PMC article.
-
Evidence that Alzheimer's Disease Is a Disease of Competitive Synaptic Plasticity Gone Awry.J Alzheimers Dis. 2024;99(2):447-470. doi: 10.3233/JAD-240042. J Alzheimers Dis. 2024. PMID: 38669548 Free PMC article. Review.
-
Circadian Changes of Dendritic Spine Geometry in Mouse Barrel Cortex.Front Neurosci. 2020 Sep 29;14:578881. doi: 10.3389/fnins.2020.578881. eCollection 2020. Front Neurosci. 2020. PMID: 33117123 Free PMC article.
References
-
- Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim K, Mundel P. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol. 2006;8:485–491. - PubMed
-
- Bas Orth C, Vlachos A, Del Turco D, Burbach GJ, Haas CA, Mundel P, Feng G, Frotscher M, Deller T. Lamina-specific distribution of synaptopodin, an actin-associated molecule essential for the spine apparatus, in identified principal cell dendrites of the mouse hippocampus. J Comp Neurol. 2005;487:227–239. - PubMed
-
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. - PubMed
-
- Burgoyne RD, Barron J, Geisow MJ. Cytochemical localisation of calcium binding sites in adrenal chromaffin cells and their relation to secretion. Cell Tissue Res. 1983;229:207–217. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
