Intracellular Zn2+ accumulation contributes to synaptic failure, mitochondrial depolarization, and cell death in an acute slice oxygen-glucose deprivation model of ischemia
- PMID: 19176819
- PMCID: PMC2637403
- DOI: 10.1523/JNEUROSCI.4604-08.2009
Intracellular Zn2+ accumulation contributes to synaptic failure, mitochondrial depolarization, and cell death in an acute slice oxygen-glucose deprivation model of ischemia
Abstract
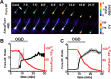
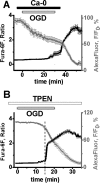
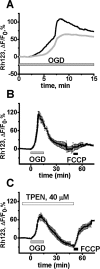
Despite considerable evidence for contributions of both Zn(2+) and Ca(2+) in ischemic brain damage, the relative importance of each cation to very early events in injury cascades is not well known. We examined Ca(2+) and Zn(2+) dynamics in hippocampal slices subjected to oxygen-glucose deprivation (OGD). When single CA1 pyramidal neurons were loaded via a patch pipette with a Ca(2+)-sensitive indicator (fura-6F) and an ion-insensitive indicator (AlexaFluor-488), small dendritic fura-6F signals were noted after several (approximately 6-8) minutes of OGD, followed shortly by sharp somatic signals, which were attributed to Ca(2+) ("Ca(2+) deregulation"). At close to the time of Ca(2+) deregulation, neurons underwent a terminal increase in plasma membrane permeability, indicated by loss of AlexaFluor-488 fluorescence. In neurons coloaded with fura-6F and a Zn(2+)-selective indicator (FluoZin-3), progressive rises in cytosolic Zn(2+) levels were detected before Ca(2+) deregulation. Addition of the Zn(2+) chelator N,N,N',N'-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) significantly delayed both Ca(2+) deregulation and the plasma membrane permeability increases, indicating that Zn(2+) contributes to the degenerative signaling. Present observations further indicate that Zn(2+) is rapidly taken up into mitochondria, contributing to their early depolarization. Also, TPEN facilitated recovery of the mitochondrial membrane potential and of field EPSPs after transient OGD, and combined removal of Ca(2+) and Zn(2+) markedly extended the duration of OGD tolerated. These data provide new clues that Zn(2+) accumulates rapidly in neurons during slice OGD, is taken up by mitochondria, and contributes to consequent mitochondrial dysfunction, cessation of synaptic transmission, Ca(2+) deregulation, and cell death.
Figures




References
-
- Arslan P, Di Virgilio F, Beltrame M, Tsien RY, Pozzan T. Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+ J Biol Chem. 1985;260:2719–2727. - PubMed
-
- Bahar S, Fayuk D, Somjen GG, Aitken PG, Turner DA. Mitochondrial and intrinsic optical signals imaged during hypoxia and spreading depression in rat hippocampal slices. J Neurophysiol. 2000;84:311–324. - PubMed
-
- Bonanni L, Chachar M, Jover-Mengual T, Li H, Jones A, Yokota H, Ofengeim D, Flannery RJ, Miyawaki T, Cho CH, Polster BM, Pypaert M, Hardwick JM, Sensi SL, Zukin RS, Jonas EA. Zinc-dependent multi-conductance channel activity in mitochondria isolated from ischemic brain. J Neurosci. 2006;26:6851–6862. - PMC - PubMed
-
- Bossy-Wetzel E, Talantova MV, Lee WD, Schölzke MN, Harrop A, Mathews E, Götz T, Han J, Ellisman MH, Perkins GA, Lipton SA. Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38-activated K(+) channels. Neuron. 2004;41:351–365. - PubMed
-
- Brown AM, Kristal BS, Effron MS, Shestopalov AI, Ullucci PA, Sheu KF, Blass JP, Cooper AJ. Zn2+ inhibits alpha-ketoglutarate-stimulated mitochondrial respiration and the isolated alpha-ketoglutarate dehydrogenase complex. J Biol Chem. 2000;275:13441–13447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
