Fgfr1 is required for cortical regeneration and repair after perinatal hypoxia
- PMID: 19176828
- PMCID: PMC2768410
- DOI: 10.1523/JNEUROSCI.4516-08.2009
Fgfr1 is required for cortical regeneration and repair after perinatal hypoxia
Abstract
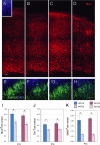
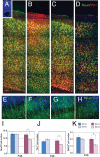
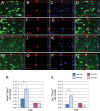
Chronic postnatal hypoxia causes an apparent loss of cortical neurons that is reversed during recovery (Fagel et al., 2006). The cellular and molecular mechanisms underlying this plasticity are not understood. Here, we show that chronic hypoxia from postnatal days 3 (P3) to 10 causes a 30% decrease in cortical neurons and a 24% decrease in cortical volume. T-brain-1 (Tbr1)(+) and SMI-32(+) excitatory neuron numbers were completely recovered 1 month after the insult, but the mice showed a residual deficit in Parvalbumin(+) and Calretinin(+) GABAergic interneurons. In contrast, hypoxic mice carrying a disrupted fibroblast growth factor receptor-1 (Fgfr1) gene in GFAP+ cells [Fgfr1 conditional knock-out (cKO)], demonstrated a persistent loss of excitatory cortical neurons and a worsening of the interneuron defect. Labeling proliferating progenitors at P17 revealed increased generation of cortical NeuN(+) and Tbr1(+) excitatory neurons in wild-type mice subjected to hypoxic insult, whereas Fgfr1 cKO failed to mount a cortical neurogenetic response. Hypoxic wild-type mice also demonstrated a twofold increase in cell proliferation in the subventricular zone (SVZ) at P17 and a threefold increase in neurogenesis in the olfactory bulb (OB) at P48, compared with normoxic mice. In contrast, Fgfr1 cKO mice had decreased SVZ cell proliferation and curtailed reactive neurogenesis in the OB. Thus, the activation of FGFR-1 in GFAP+ cells is required for neuronal recovery after neonatal hypoxic injury, which is attributable in part to enhanced cortical and OB neurogenesis. In contrast, there is incomplete recovery of inhibitory neurons after injury, which may account for persistent behavioral deficits.
Figures


References
-
- Allin M, Henderson M, Suckling J, Nosarti C, Rushe T, Fearon P, Stewart AL, Bullmore ET, Rifkin L, Murray R. Effects of very low birthweight on brain structure in adulthood. Dev Med Child Neurol. 2004;46:46–53. - PubMed
-
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. - PubMed
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
