Network architecture of gap junction-coupled neuronal linkage in the striatum
- PMID: 19176831
- PMCID: PMC6665140
- DOI: 10.1523/JNEUROSCI.4418-08.2009
Network architecture of gap junction-coupled neuronal linkage in the striatum
Abstract
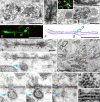
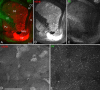
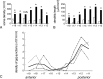
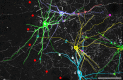
Previous studies have revealed the existence of gap junctions between GABAergic interneurons of a particular type in the striatum. Because of the technical difficulties, however, there is no information about their positions within the striatal circuitry. We have developed a method to detect neuronal gap junctions reliably at the light microscopic level and thereby explored the network architecture of the gap junctional linkage. Gap junction-coupled networks among parvalbumin-containing GABAergic interneurons extended nonuniformly in the feline striatum. They were located predominantly in the methionine-enkephalin-poor matrix. Moreover, the density of gap junctional coupling showed a marked regional difference along the anterior-posterior axis of the striatum. The densest interconnectivity was found in the posterior part of both caudate nucleus and putamen that corresponds to the sensory-recipient area of the feline striatum. Electron microscopic observations provided clear evidence of internalization of neuronal gap junction, indicating the dynamic nature of gap junctional linkage between neurons in vivo. The nonuniform organization of gap junction networks suggests differential modes of information processing in heterogeneous subregions of the striatum.
Figures


Similar articles
-
Gap junctions among dendrites of cortical GABAergic neurons establish a dense and widespread intercolumnar network.J Neurosci. 2006 Mar 29;26(13):3434-43. doi: 10.1523/JNEUROSCI.4076-05.2006. J Neurosci. 2006. PMID: 16571750 Free PMC article.
-
Ultrastructural study of gap junctions between dendrites of parvalbumin-containing GABAergic neurons in various neocortical areas of the adult rat.Neuroscience. 2003;120(1):5-20. doi: 10.1016/s0306-4522(03)00328-2. Neuroscience. 2003. PMID: 12849736
-
Structural organization of the gap junction network in the cerebral cortex.Neuroscientist. 2007 Jun;13(3):199-207. doi: 10.1177/1073858406296760. Neuroscientist. 2007. PMID: 17519363 Review.
-
Distal gap junctions and active dendrites can tune network dynamics.J Neurophysiol. 2006 Mar;95(3):1669-82. doi: 10.1152/jn.00662.2005. Epub 2005 Dec 7. J Neurophysiol. 2006. PMID: 16339003
-
Control of gap-junctional communication in astrocytic networks.Trends Neurosci. 1996 Aug;19(8):319-25. doi: 10.1016/0166-2236(96)10046-1. Trends Neurosci. 1996. PMID: 8843600 Review.
Cited by
-
Striatal interneurons in dissociated cell culture.Histochem Cell Biol. 2010 Jul;134(1):1-12. doi: 10.1007/s00418-010-0707-9. Epub 2010 May 19. Histochem Cell Biol. 2010. PMID: 20490535 Free PMC article.
-
Dysregulation of Corticostriatal Connectivity in Huntington's Disease: A Role for Dopamine Modulation.J Huntingtons Dis. 2016 Dec 15;5(4):303-331. doi: 10.3233/JHD-160221. J Huntingtons Dis. 2016. PMID: 27983564 Free PMC article. Review.
-
Local dynamics of gap-junction-coupled interneuron networks.Phys Biol. 2010 Mar 12;7:16015. doi: 10.1088/1478-3975/7/1/016015. Phys Biol. 2010. PMID: 20228446 Free PMC article.
-
The computational power of the human brain.Front Cell Neurosci. 2023 Aug 7;17:1220030. doi: 10.3389/fncel.2023.1220030. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37608987 Free PMC article. Review.
-
Dynamics of action potential firing in electrically connected striatal fast-spiking interneurons.Front Cell Neurosci. 2013 Nov 14;7:209. doi: 10.3389/fncel.2013.00209. eCollection 2013. Front Cell Neurosci. 2013. PMID: 24294191 Free PMC article.
References
-
- Bennett BD, Bolam JP. Localisation of parvalbumin-immunoreactive structures in primate caudate-putamen. J Comp Neurol. 1994a;347:340–356. - PubMed
-
- Bennett BD, Bolam JP. Synaptic input and output of parvalbumin-immunoreactive neurons in the neostriatum of the rat. Neuroscience. 1994b;62:707–719. - PubMed
-
- Condorelli DF, Parenti R, Spinella F, Trovato Salinaro A, Belluardo N, Cardile V, Cicirata F. Cloning of a new gap junction gene (CX36) highly expressed in mammalian brain neurons. Eur J Neurosci. 1998;10:1202–1208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
