Steroid interaction with a single potentiating site is sufficient to modulate GABA-A receptor function
- PMID: 19176850
- PMCID: PMC2684936
- DOI: 10.1124/mol.108.053629
Steroid interaction with a single potentiating site is sufficient to modulate GABA-A receptor function
Abstract
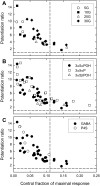
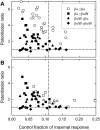
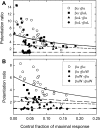
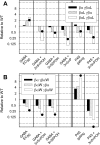
Neuroactive steroids are efficacious potentiators of GABA-A receptors. Recent work has identified a site in the alpha1 subunit of the GABA-A receptor, that is essential for potentiation by steroids. However, each receptor contains two copies of the alpha1 subunit. We generated concatemers of subunits so that the alpha1 subunits could be mutated separately and examined the consequences of mutations that remove potentiation by most neurosteroids (alpha1 Q241L, alpha1 Q241W). Concatemers were expressed in Xenopus laevis oocytes, and activation by GABA, potentiation by neurosteroids, and the agonist activity of piperidine-4-sulfonic acid (P4S) were determined. When the alpha1 Q241L mutation is present in alpha1 subunits the EC(50) for activation by GABA is shifted to higher concentration and potentiation by neurosteroids is diminished. When the alpha1 Q241W mutation is expressed, the EC(50) for GABA is shifted to lower concentration, the relative efficacy of P4S is increased, and potentiation by neurosteroids is diminished. Mutation of only one alpha1 subunit does not produce the full effect of mutating both sites. Overall, the data demonstrate that at a macroscopic level, the presence of a single wild-type steroid-binding site is sufficient to mediate responses to steroid, but both must be mutated to completely remove the effects of steroids. Differences between the two sites seem to be relatively subtle.
Figures




References
-
- Baumann SW, Baur R, and Sigel E (2001) Subunit arrangement of γ-aminobutyric acid type A receptors. J Biol Chem 276 36275-36280. - PubMed
-
- Baumann SW, Baur R, and Sigel E (2002) Forced subunit assembly in α1β2γ2 GABA-A receptors. Insight into the absolute arrangement. J Biol Chem 277 46020-46025. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

