Estradiol suppresses glutamatergic transmission to gonadotropin-releasing hormone neurons in a model of negative feedback in mice
- PMID: 19176881
- PMCID: PMC2804800
- DOI: 10.1095/biolreprod.108.075077
Estradiol suppresses glutamatergic transmission to gonadotropin-releasing hormone neurons in a model of negative feedback in mice
Abstract
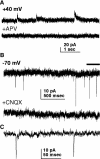
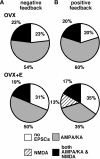
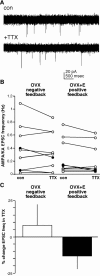
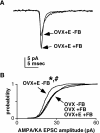
A surge of gonadotropin-releasing hormone (GnRH) release from the brain triggers the luteinizing hormone (LH) surge that causes ovulation. The GnRH surge is initiated by a switch in estradiol action from negative to positive feedback. Estradiol signals critical for the surge are likely transmitted to GnRH neurons at least in part via estradiol-sensitive afferents. Using an ovariectomized estradiol-treated (OVX+E) mouse model that exhibits daily LH surges, we examined changes in glutamate transmission to GnRH neurons during negative feedback and positive feedback. Spontaneous glutamatergic excitatory postsynaptic currents (EPSCs) mediated by alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid/kainate receptors (AMPA/KA Rs) or N-methyl-D-aspartate receptors (NMDARs) were recorded in GnRH neurons from OVX+E and OVX mice. There were no diurnal changes in the percentage of GnRH neurons from OVX mice exhibiting EPSCs. In cells from OVX+E mice, the profile of AMPA/KA R-mediated and NMDAR-mediated EPSCs showed changes dependent on time of day. Comparison of AMPA/KA R-mediated EPSC frequency in OVX+E and OVX cells showed that estradiol suppressed transmission during negative feedback but had no effect during positive feedback. Tetrodotoxin treatment to block action potential firing did not affect AMPA/KA R-mediated EPSC frequency in OVX cells during negative feedback or in OVX+E cells during positive feedback, suggesting that estradiol-induced suppression of glutamate transmission may be primarily due to activity-independent changes. The diurnal removal of estradiol-induced suppression of AMPA/KA R-mediated glutamate transmission to GnRH neurons during positive feedback suggests that the primary role for estradiol-induced changes in glutamate transmission may be in mediating negative feedback.
Figures

Similar articles
-
Critical roles for fast synaptic transmission in mediating estradiol negative and positive feedback in the neural control of ovulation.Endocrinology. 2008 Nov;149(11):5500-8. doi: 10.1210/en.2008-0453. Epub 2008 Jul 10. Endocrinology. 2008. PMID: 18617615 Free PMC article.
-
Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation.J Neurosci. 2007 Feb 21;27(8):1913-21. doi: 10.1523/JNEUROSCI.4738-06.2007. J Neurosci. 2007. PMID: 17314287 Free PMC article.
-
Changes in Both Neuron Intrinsic Properties and Neurotransmission Are Needed to Drive the Increase in GnRH Neuron Firing Rate during Estradiol-Positive Feedback.J Neurosci. 2019 Mar 13;39(11):2091-2101. doi: 10.1523/JNEUROSCI.2880-18.2019. Epub 2019 Jan 17. J Neurosci. 2019. PMID: 30655354 Free PMC article.
-
The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges.Endocr Rev. 2010 Aug;31(4):544-77. doi: 10.1210/er.2009-0023. Epub 2010 Mar 17. Endocr Rev. 2010. PMID: 20237240 Free PMC article. Review.
-
Excitatory amino acids: function and significance in reproduction and neuroendocrine regulation.Front Neuroendocrinol. 1994 Mar;15(1):3-49. doi: 10.1006/frne.1994.1002. Front Neuroendocrinol. 1994. PMID: 7958168 Review.
Cited by
-
Circadian control of neuroendocrine circuits regulating female reproductive function.Front Endocrinol (Lausanne). 2012 May 21;3:60. doi: 10.3389/fendo.2012.00060. eCollection 2012. Front Endocrinol (Lausanne). 2012. PMID: 22661968 Free PMC article.
-
Orexin a suppresses gonadotropin-releasing hormone (GnRH) neuron activity in the mouse.Endocrinology. 2012 Aug;153(8):3850-60. doi: 10.1210/en.2012-1300. Epub 2012 Jun 6. Endocrinology. 2012. PMID: 22673226 Free PMC article.
-
GABAergic transmission to gonadotropin-releasing hormone (GnRH) neurons is regulated by GnRH in a concentration-dependent manner engaging multiple signaling pathways.J Neurosci. 2009 Aug 5;29(31):9809-18. doi: 10.1523/JNEUROSCI.2509-09.2009. J Neurosci. 2009. PMID: 19657033 Free PMC article.
-
Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype.J Neurosci. 2009 Apr 29;29(17):5616-27. doi: 10.1523/JNEUROSCI.0352-09.2009. J Neurosci. 2009. PMID: 19403828 Free PMC article.
-
Continuous Kisspeptin Administration in Postmenopausal Women: Impact of Estradiol on Luteinizing Hormone Secretion.J Clin Endocrinol Metab. 2017 Jun 1;102(6):2091-2099. doi: 10.1210/jc.2016-3952. J Clin Endocrinol Metab. 2017. PMID: 28368443 Free PMC article.
References
-
- Docke F, Dorner G.The mechanism of the induction of ovulation by oestrogens. J Endocrinol 1965; 33: 491–499. - PubMed
-
- Sarkar DK, Fink G.Luteinizing hormone releasing factor in pituitary stalk plasma from long-term ovariectomized rats: effects of steroids. J Endocrinol 1980; 86: 511–524. - PubMed
-
- Karsch FJ.Central actions of ovarian steroids in the feedback regulation of pulsatile secretion of luteinizing hormone. Ann Rev Physiol 1987; 49: 365–382. - PubMed
-
- Herbison AE.Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev 1998; 19: 302–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

