Diminished presynaptic GABA(B) receptor function in the neocortex of a genetic model of absence epilepsy
- PMID: 19176980
- PMCID: PMC4878904
- DOI: 10.1159/000197864
Diminished presynaptic GABA(B) receptor function in the neocortex of a genetic model of absence epilepsy
Abstract
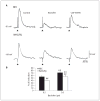
Changes in GABA(B) receptor subunit expression have been recently reported in the neocortex of epileptic WAG/Rij rats that are genetically prone to experience absence seizures. These alterations may lead to hyperexcitability by downregulating the function of presynaptic GABA(B) receptors in neocortical networks as suggested by a reduction in paired-pulse depression. Here, we tested further this hypothesis by analyzing the effects induced by the GABA(B) receptor agonist baclofen (0.1-10 microM) on the inhibitory events recorded in vitro from neocortical slices obtained from epileptic (>180 day-old) WAG/Rij and age-matched, non-epileptic control (NEC) rats. We found that higher doses of baclofen were required to depress pharmacologically isolated, stimulus-induced IPSPs generated by WAG/Rij neurons as compared to NEC. We also obtained similar evidence by comparing the effects of baclofen on the rate of occurrence of synchronous GABAergic events recorded by WAG/Rij and NEC neocortical slices treated with 4-aminopyridine + glutamatergic receptor antagonists. In conclusion, these data highlight a decreased function of presynaptic GABA(B) receptors in the WAG/Rij rat neocortex. We propose that this alteration may contribute to neocortical hyperexcitability and thus to absence seizures.
2009 S. Karger AG, Basel.
Figures




Similar articles
-
Reduced GABAB receptor subunit expression and paired-pulse depression in a genetic model of absence seizures.Neurobiol Dis. 2007 Mar;25(3):631-41. doi: 10.1016/j.nbd.2006.11.005. Epub 2007 Jan 3. Neurobiol Dis. 2007. PMID: 17207629
-
Neocortical hyperexcitability in a genetic model of absence seizures and its reduction by levetiracetam.Epilepsia. 2006 Jul;47(7):1144-52. doi: 10.1111/j.1528-1167.2006.00588.x. Epilepsia. 2006. PMID: 16886977
-
Regulation of synaptic input to hypothalamic presympathetic neurons by GABA(B) receptors.Neuroscience. 2006 Oct 13;142(2):595-606. doi: 10.1016/j.neuroscience.2006.06.039. Epub 2006 Aug 2. Neuroscience. 2006. PMID: 16887273
-
Synaptic hyperexcitability of deep layer neocortical cells in a genetic model of absence seizures.Genes Brain Behav. 2006 Feb;5(1):73-84. doi: 10.1111/j.1601-183X.2005.00146.x. Genes Brain Behav. 2006. PMID: 16436191
-
The physiological role of pre- and postsynaptic GABA(B) receptors in membrane excitability and synaptic transmission of neurons in the rat's dorsal cortex of the inferior colliculus.Neuroscience. 2009 Apr 21;160(1):198-211. doi: 10.1016/j.neuroscience.2009.02.011. Neuroscience. 2009. PMID: 19409201
Cited by
-
Dynamics of networks during absence seizure's on- and offset in rodents and man.Front Physiol. 2015 Feb 5;6:16. doi: 10.3389/fphys.2015.00016. eCollection 2015. Front Physiol. 2015. PMID: 25698972 Free PMC article. Review.
-
Impact of strain, sex, and estrous cycle on gamma butyrolactone-evoked absence seizures in rats.Epilepsy Res. 2018 Nov;147:62-70. doi: 10.1016/j.eplepsyres.2018.09.007. Epub 2018 Sep 18. Epilepsy Res. 2018. PMID: 30261353 Free PMC article.
-
Rhythm and blues: animal models of epilepsy and depression comorbidity.Biochem Pharmacol. 2013 Jan 15;85(2):135-46. doi: 10.1016/j.bcp.2012.08.016. Epub 2012 Aug 23. Biochem Pharmacol. 2013. PMID: 22940575 Free PMC article. Review.
-
The impact of early-life environment on absence epilepsy and neuropsychiatric comorbidities.IBRO Neurosci Rep. 2022 Nov 9;13:436-468. doi: 10.1016/j.ibneur.2022.10.012. eCollection 2022 Dec. IBRO Neurosci Rep. 2022. PMID: 36386598 Free PMC article. Review.
-
Separating Neural Oscillations from Aperiodic 1/f Activity: Challenges and Recommendations.Neuroinformatics. 2022 Oct;20(4):991-1012. doi: 10.1007/s12021-022-09581-8. Epub 2022 Apr 7. Neuroinformatics. 2022. PMID: 35389160 Free PMC article.
References
-
- Blumenfeld H. Cellular and network mechanisms of spike-wave seizures. Epilepsia. 2005;46(suppl 9):21–33. - PubMed
-
- Crunelli V, Leresche N. Childhood absence epilepsy: genes, channels, neurons and networks. Nat Rev Neurosci. 2002;3:371–382. - PubMed
-
- Klein JP, Khera DS, Nersesyan H, Kimchi EY, Waxman SG, Blumenfeld H. Dysregulation of sodium channel expression in cortical neurons in a rodent model of absence epilepsy. Brain Res. 2004;1000:102–109. - PubMed
-
- Strauss U, Kole MH, Brauer AU, Pahnke J, Bajorat R, Rolfs A, Nitsch R, Deisz RA. An impaired neocortical Ih is associated with enhanced excitability and absence epilepsy. Eur J Neurosci. 2004;19:3048–3058. - PubMed
-
- D’Antuono M, Inaba Y, Biagini G, D’Arc-angelo G, Tancredi V, Avoli M. Synaptic hyperexcitability of deep layer neocortical cells in a genetic model of absence seizures. Genes Brain Behav. 2006;5:73–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

