Enhanced sensitivity of the MRL/MpJ mouse to the neuroplastic and behavioral effects of chronic antidepressant treatments
- PMID: 19177066
- PMCID: PMC2680932
- DOI: 10.1038/npp.2008.234
Enhanced sensitivity of the MRL/MpJ mouse to the neuroplastic and behavioral effects of chronic antidepressant treatments
Abstract
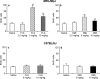
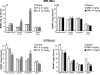
Chronic administration of antidepressant drugs produce changes in neuroplasticity and behavior in rodents, effects that may be associated with the slow emergence of clinical therapeutic effects. Owing to the uncertainty over the effects of chronic antidepressant treatments in mice, these experiments compared the regulation of neurogenesis, neurotrophin levels, and behavior produced by chronic antidepressant treatments between two inbred mouse strains, MRL/MpJ and C57BL/6J. The MRL/MpJ strain is associated with enhanced wound healing and tissue regeneration, whereas C57BL/6J mice are used commonly for behavioral studies. Proliferation and survival of hippocampal progenitor cells were measured using flow cytometry, a new platform that rapidly quantifies the incorporation of 5-bromo-2-deoxyuridine (BrdU). Hippocampal cell proliferation was increased significantly after chronic administration of fluoxetine (FLX: 5, 10 mg/kg, intraperitoneal (i.p.), b.i.d.) or desipramine (DMI: 5, 10 mg/kg, i.p., b.i.d.) for 21 days in MRL/MpJ mice, but not in C57BL/6J mice. Hippocampal progenitor cells born prior to chronic antidepressant treatments were not affected in either mouse strain. Protein levels of brain-derived neurotrophic factor (BDNF) in MRL/MpJ mice were elevated significantly in the frontal cortex, hippocampus, and amygdala after chronic FLX treatment, but increased only in the frontal cortex by chronic DMI. In contrast, BDNF levels in C57BL/6J mice were decreased in the hippocampus and increased in the amygdala after chronic FLX, and were decreased in the brain stem after chronic DMI. Novelty-induced hypophagia (NIH) was used to examine a behavioral effect produced by chronic antidepressant treatment. MRL/MpJ mice, chronically administered FLX or DMI, had significantly shorter latencies to consume food when exposed to a novel environment than untreated mice, whereas there were no effects on the behavior of C57BL/6J mice. In conclusion, robust effects of chronic antidepressant treatments on hippocampal cell proliferation and BDNF levels paralleled the ability of these drugs to produce changes in NIH behavior in MRL/MpJ, while none of these effects were produced in C57BL/6J mice. The greater responsiveness of MRL/MpJ mice may be important for drug discovery, for genetic studies, and for understanding the neural mechanisms underlying the physiological and behavioral effects of chronic antidepressant treatments.
Figures



References
-
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. - PubMed
-
- Altar CA, Whitehead RE, Chen R, Wortwein G, Madsen TM. Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol Psychiatry. 2003;54:703–709. - PubMed
-
- Baker KL, Daniels SB, Lennington JB, Lardaro T, Czap A, Notti RQ, Cooper O, Isacson O, Frasca S, Jr., Conover JC. Neuroblast protuberances in the subventricular zone of the regenerative MRL/MpJ mouse. J Comp Neurol. 2006;498:747–761. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

