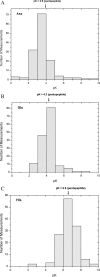
A summary of the measured pK values of the ionizable groups in folded proteins
- PMID: 19177368
- PMCID: PMC2708032
- DOI: 10.1002/pro.19
A summary of the measured pK values of the ionizable groups in folded proteins
Abstract
We tabulated 541 measured pK values reported in the literature for the Asp, Glu, His, Cys, Tyr, and Lys side chains, and the C and N termini of 78 folded proteins. The majority of these values are for the Asp, Glu, and His side chains. The average pK values are Asp 3.5 +/- 1.2 (139); Glu 4.2 +/- 0.9 (153); His 6.6 +/- 1.0 (131); Cys 6.8 +/- 2.7 (25); Tyr 10.3 +/- 1.2 (20); Lys 10.5 +/- 1.1 (35); C-terminus 3.3 +/- 0.8 (22) and N-terminus 7.7 +/- 0.5 (16). We compare these results with the measured pK values of these groups in alanine pentapeptides, and comment on our overall findings.
Figures
References
-
- Nozaki Y, Tanford C. Examination of titration behavior. Methods Enzymol. 1967;11:715–734.
-
- Cho JH, Raleigh DP. Mutational analysis demonstrates that specific electrostatic interactions can play a key role in the denatured state ensemble of proteins. J Mol Biol. 2005;353:174–185. - PubMed
-
- Marti DN. Apparent pKa shifts of titratable residues at high denaturant concentration and the impact on protein stability. Biophys Chem. 2005;118:88–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources