Walker-A threonine couples nucleotide occupancy with the chaperone activity of the AAA+ ATPase ClpB
- PMID: 19177562
- PMCID: PMC2708065
- DOI: 10.1002/pro.36
Walker-A threonine couples nucleotide occupancy with the chaperone activity of the AAA+ ATPase ClpB
Abstract
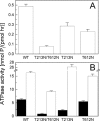
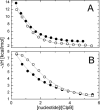
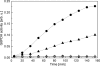
Hexameric AAA+ ATPases induce conformational changes in a variety of macromolecules. AAA+ structures contain the nucleotide-binding P-loop with the Walker A sequence motif: GxxGxGK(T/S). A subfamily of AAA+ sequences contains Asn in the Walker A motif instead of Thr or Ser. This noncanonical subfamily includes torsinA, an ER protein linked to human dystonia and DnaC, a bacterial helicase loader. Role of the noncanonical Walker A motif in the functionality of AAA+ ATPases has not been explored yet. To determine functional effects of introduction of Asn into the Walker A sequence, we replaced the Walker-A Thr with Asn in ClpB, a bacterial AAA+ chaperone which reactivates aggregated proteins. We found that the T-to-N mutation in Walker A partially inhibited the ATPase activity of ClpB, but did not affect the ClpB capability to associate into hexamers. Interestingly, the noncanonical Walker A sequence in ClpB induced preferential binding of ADP vs. ATP and uncoupled the linkage between the ATP-bound conformation and the high-affinity binding to protein aggregates. As a consequence, ClpB with the noncanonical Walker A sequence showed a low chaperone activity in vitro and in vivo. Our results demonstrate a novel role of the Walker-A Thr in sensing the nucleotide's gamma-phosphate and in maintaining an allosteric linkage between the P-loop and the aggregate binding site of ClpB. We postulate that AAA+ ATPases with the noncanonical Walker A might utilize distinct mechanisms to couple the ATPase cycle with their substrate-remodeling activity.
Figures


References
-
- Neuwald AF. Aravind L. Spouge JL. Koonin EV. AAA+: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9:27–43. - PubMed
-
- Hanson PI. Whiteheart SW. AAA+ proteins: have engine, will work. Nat Rev Mol Cell Biol. 2005;6:519–529. - PubMed
-
- Ogura T. Wilkinson AJ. AAA+ superfamily ATPases: common structure–diverse function. Genes Cells. 2001;6:575–597. - PubMed
-
- Weibezahn J. Schlieker C. Tessarz P. Mogk A. Bukau B. Novel insights into the mechanism of chaperone-assisted protein disaggregation. Biol Chem. 2005;386:739–744. - PubMed
-
- Lee S. Sowa ME. Watanabe YH. Sigler PB. Chiu W. Yoshida M. Tsai FT. The structure of ClpB: a molecular chaperone that rescues proteins from an aggregated state. Cell. 2003;115:229–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

