Design of a heterotetrameric coiled coil
- PMID: 19177574
- PMCID: PMC2708066
- DOI: 10.1002/pro.30
Design of a heterotetrameric coiled coil
Abstract
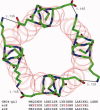
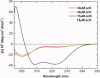
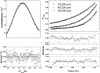
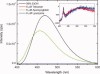
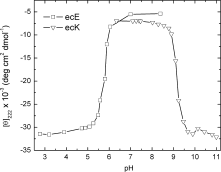
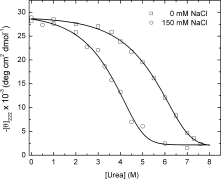
We have successfully designed a simple peptide sequence that forms highly stable coiled-coil heterotetramers. Our model system is based on the GCN4-pLI parallel coiled-coil tetramer, first described by Kim and coworkers (Harbury et al., Science 1993;262:1401-1407). We introduced glutamates at all of the e and c heptad positions of one sequence (ecE) and lysines at the same positions in a second sequence (ecK). Based on a modeling study, these sidechains are close enough in space to form structure-stabilizing salt bridges. We show that ecE and ecK are highly unstable by themselves but form very stable parallel helical tetramers when mixed, as judged by circular dichroism, analytical ultracentrifugation, and disulfide crosslinking studies. The origin of the difference in stabilities between the homomeric structures and the heteromeric structures comes from a combination of the relief of electrostatic repulsions with concomitant formation of electrostatic attractive interactions based on pH and NaCl screening experiments. We quantify the stability of the heterotetrameric coiled coil from a thermodynamic analysis and compare the finding to other similar coiled-coil systems.
Figures
References
-
- Fairman R, Akerfeldt KS. Peptides as novel smart materials. Curr Opin Struct Biol. 2005;15:453–463. - PubMed
-
- Woolfson DN. The design of coiled-coil structures and assemblies. Adv Protein Chem. 2005;70:79–112. - PubMed
-
- Woolfson DN, Ryadnov MG. Peptide-based fibrous biomaterials: some things old, new and borrowed. Curr Opin Chem Biol. 2006;10:559–567. - PubMed
-
- Fairman R, Chao HG, Lavoie TB, Villafranca JJ, Matsueda GR, Novotny J. Design of heterotetrameric coiled coils: evidence for increased stabilization by Glu(−)-Lys(+) ion pair interactions. Biochemistry. 1996;35:2824–2829. - PubMed
-
- Lumb KJ, Kim PS. A buried polar interaction imparts structural uniqueness in a designed heterodimeric coiled coil. Biochemistry. 1995;34:8642–8648. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

