Characterization of a heparin-binding site on the catalytic domain of factor XIa: mechanism of heparin acceleration of factor XIa inhibition by the serpins antithrombin and C1-inhibitor
- PMID: 19178150
- PMCID: PMC2654577
- DOI: 10.1021/bi802298r
Characterization of a heparin-binding site on the catalytic domain of factor XIa: mechanism of heparin acceleration of factor XIa inhibition by the serpins antithrombin and C1-inhibitor
Abstract
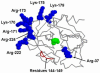
Heparin accelerates inhibition of factor XIa (fXIa) by the serpins antithrombin (AT) and C1-inhibitor (C1-INH) by more than 2 orders of magnitude. The mechanism of the heparin-mediated acceleration of fXIa inhibition by these serpins is incompletely understood, as heparin appears to interact with both the catalytic and noncatalytic domains of the protease. We replaced the basic residues of the fXIa 170 loop (Lys-170, Arg-171, Arg-173, Lys-175, and Lys-179; chymotrypsin numbering) with Ala, using an expression system that allows separation of the fXIa catalytic domain (CD) from noncatalytic domains. Heparin-mediated inhibition of 170 loop CD variants with AT was impaired 3-10-fold relative to that of the wild-type (CD-WT). In reactions with C1-INH, Arg-171 was the most critical residue contributing approximately 2-3-fold to heparin-mediated inhibition of CD-WT. A template mechanism did not fully account for the effect of heparin with either serpin, as the second-order inhibition rate constants did not exhibit a characteristic bell-shaped dependence on heparin concentration. Further studies revealed that the C1-INH inhibition of full-length fXIa containing Ala substitutions for basic residues of the 148 loop is not enhanced by heparin. Inhibition by AT of a full-length fXIa variant containing an Ala substitution for Arg-37 in the fXIa CD was approximately 5-fold greater than for wild-type fXIa in the absence of heparin. These results suggest that basic residues of the fXIa 170 loop form a heparin-binding site and that the accelerating effect of heparin on inhibition of fXIa by AT or C1-INH may be mediated by charge neutralization and/or allosteric mechanisms that overcome the repulsive inhibitory interactions of serpins with basic residues on the fXIa 148 and 37 loops.
Figures




References
-
- Walsh PN, Griffin JH. Contributions of human platelets to the proteolytic activation of blood coagulation factors XII and XI. Blood. 1981;57:106–118. - PubMed
-
- Kurachi K, Davie EW. Activation of human factor XI (plasma thromboplastin antecedent) by factor XIIa (activated Hageman factor) Biochemistry. 1977;16:5831–5839. - PubMed
-
- Gailani D, Broze GJ., Jr. Factor XI activation in a revised model of blood coagulation. Science. 1991;253:909–912. - PubMed
-
- Ragni MV, Sinha D, Seaman F, Lewis JH, Spero JA, Walsh PN. Comparison of bleeding tendency, factor XI coagulant activity, and factor XI antigen in 25 factor XI-deficient kindreds. Blood. 1985;65:719–724. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

