Breaking tolerance to self, circulating natural killer cells expressing inhibitory KIR for non-self HLA exhibit effector function after T cell-depleted allogeneic hematopoietic cell transplantation
- PMID: 19179302
- PMCID: PMC2670800
- DOI: 10.1182/blood-2008-09-177055
Breaking tolerance to self, circulating natural killer cells expressing inhibitory KIR for non-self HLA exhibit effector function after T cell-depleted allogeneic hematopoietic cell transplantation
Abstract
Alloreactive natural killer (NK) cells are an important influence on hematopoietic stem cell transplantation (HSCT) outcome. In HLA-mismatched HSCT, alloreactivity occurs when licensed donor NK cells expressing inhibitory killer Ig-like receptors (KIR) for donor MHC class I ligands recognize the lack of the class I ligands in the mismatched recipient ("missing self"). Studies in HLA-matched HSCT, however, have also demonstrated improved outcome in patients lacking class I ligands for donor inhibitory KIR ("missing ligand"), indicating that classically nonlicensed donor NK cells expressing KIR for non-self MHC class I ligands may exhibit functional competence in HSCT. We examined NK function in 16 recipients of T cell-depleted allografts from HLA-identical or KIR-ligand matched donors after myeloablative therapy. After HSCT, nonlicensed NK cells expressing inhibitory KIR for non-self class I exhibit robust intracellular IFN-gamma and cytotoxic response to target cells lacking cognate ligand, gradually becoming tolerized to self by day 100. These findings could not be correlated with cytokine environment or phenotypic markers of NK development, nor could they be attributed to non-KIR receptors such as CD94/NKG2A. These findings confirm that NK alloreactivity can occur in HLA-matched HSCT, where tolerance to self is either acquired by the stem cell-derived NK cell after exiting the bone marrow or where tolerance to self can be temporarily overcome.
Figures
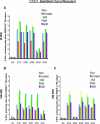

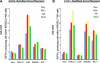
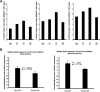


References
-
- Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. - PubMed
-
- Ljunggren HG, Karre K. In search of the missing self: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–244. - PubMed
-
- Colonna M, Samaridis J. Cloning of immunoglobulin-superfamily members associated with HLA-C and HLA-B recognition by human natural killer cells. Science. 1995;268:405–408. - PubMed
-
- Long EO. Regulation of immune responses through inhibitory receptors. Annu Rev Immunol. 1999;17:875–904. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

