Helicobacter pylori-induced interleukin-12 p40 expression
- PMID: 19179414
- PMCID: PMC2663134
- DOI: 10.1128/IAI.01456-08
Helicobacter pylori-induced interleukin-12 p40 expression
Retraction in
-
Retraction. Helicobacter pylori-induced interleukin-12 p40 expression.Infect Immun. 2011 Jan;79(1):546. doi: 10.1128/IAI.01069-10. Infect Immun. 2011. PMID: 21177935 Free PMC article. No abstract available.
Abstract
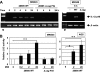
Interleukin-12 (IL-12) is a heterodimeric cytokine produced by antigen-presenting cells that promotes the development of T-helper lymphocyte 1 (Th1). Chronic gastritis induced by Helicobacter pylori is considered a Th1-mediated process. IL-12 levels in gastric biopsy samples of H. pylori-infected patients are higher than in those of uninfected individuals, but the cellular source of IL-12 remains elusive. IL-12 staining was detected in mucosal epithelial cells, lymphocytes, and macrophages in specimens of patients with H. pylori-positive gastritis. Therefore, we investigated IL-12 p40 mRNA induction by H. pylori in gastric epithelial cells and T cells. Although cag pathogenicity island (PAI)-positive H. pylori induced IL-12 p40 mRNA expression, an isogenic mutant of the cag PAI failed to induce it in both cell types. Supernatants from H. pylori cultures and H. pylori VacA induced IL-12 p40 mRNA expression in T cells but not in epithelial cells. The activation of the IL-12 p40 promoter by H. pylori was mediated through NF-kappaB. The transfection of IkappaB kinase and NF-kappaB-inducing kinase dominant-negative mutants inhibited H. pylori-induced IL-12 p40 activation. Inhibitors of NF-kappaB, phosphatidylinositol 3-kinase, p38 mitogen-activated protein kinase, and Hsp90 suppressed H. pylori- and VacA-induced IL-12 p40 mRNA expression. The results indicate that H. pylori induces IL-12 p40 expression by the activation of NF-kappaB, phosphatidylinositol 3-kinase, and p38 mitogen-activated protein kinase. Hsp90 is also a crucial regulator of H. pylori-induced IL-12 p40 expression. In addition to the cag PAI, VacA might be relevant in the induction of IL-12 expression and a Th1-polarized response only in T cells.
Figures


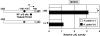




References
-
- Akopyants, N. S., S. W. Clifton, D. Kersulyte, J. E. Crabtree, B. E. Youree, C. A. Reece, N. O. Bukanov, E. S. Drazek, B. A. Roe, and D. E. Berg. 1998. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol. Microbiol. 2837-53. - PubMed
-
- Baldari, C. T., A. Lanzavecchia, and J. L. Telford. 2005. Immune subversion by Helicobacter pylori. Trends Immunol. 26199-207. - PubMed
-
- Bhat, N. R., D. L. Feinstein, Q. Shen, and A. N. Bhat. 2002. p38 MAPK-mediated transcriptional activation of inducible nitric-oxide synthase in glial cells: roles of nuclear factors, nuclear factor κB, cAMP response element-binding protein, CCAAT/enhancer-binding protein-β, and activating transcription factor-2. J. Biol. Chem. 27729584-29592. - PubMed
-
- Blaser, M. J., G. I. Perez-Perez, H. Kleanthous, T. L. Cover, R. M. Peek, P. H. Chyou, G. N. Stemmermann, and A. Nomura. 1995. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 552111-2115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

