Role of estradiol in cortisol-induced reduction of luteinizing hormone pulse frequency
- PMID: 19179435
- PMCID: PMC2689807
- DOI: 10.1210/en.2008-1754
Role of estradiol in cortisol-induced reduction of luteinizing hormone pulse frequency
Abstract
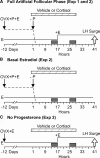
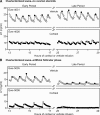
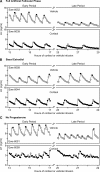
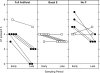
Precise control of pulsatile GnRH and LH release is imperative to ovarian cyclicity but is vulnerable to environmental perturbations, like stress. In sheep, a sustained (29 h) increase in plasma cortisol to a level observed during stress profoundly reduces GnRH pulse frequency in ovariectomized ewes treated with ovarian steroids, whereas shorter infusion (6 h) is ineffective in the absence of ovarian hormones. This study first determined whether the ovarian steroid milieu or duration of exposure is the relevant factor in determining whether cortisol reduces LH pulse frequency. Prolonged (29 h) cortisol infusion did not lower LH pulse frequency in ovariectomized ewes deprived of ovarian hormones, but it did so in ovariectomized ewes treated with estradiol and progesterone to create an artificial estrous cycle, implicating ovarian steroids as the critical factor. Importantly, this effect of cortisol was more pronounced after the simulated preovulatory estradiol rise of the artificial follicular phase. The second experiment examined which component of the ovarian steroid milieu enables cortisol to reduce LH pulse frequency in the artificial follicular phase: prior exposure to progesterone in the luteal phase, low early follicular phase estradiol levels, or the preovulatory estradiol rise. Basal estradiol enabled cortisol to decrease LH pulse frequency, but the response was potentiated by the estradiol rise. These findings lead to the conclusion that ovarian steroids, particularly estradiol, enable cortisol to inhibit LH pulse frequency. Moreover, the results provide new insight into the means by which gonadal steroids, and possibly reproductive status, modulate neuroendocrine responses to stress.
Figures
Similar articles
-
Cortisol reduces gonadotropin-releasing hormone pulse frequency in follicular phase ewes: influence of ovarian steroids.Endocrinology. 2009 Jan;150(1):341-9. doi: 10.1210/en.2008-0587. Epub 2008 Sep 18. Endocrinology. 2009. PMID: 18801903 Free PMC article.
-
Effect of transport on pulsatile LH release in ovariectomized ewes with or without prior steroid exposure at different times of year.J Reprod Fertil. 1999 Nov;117(2):213-22. doi: 10.1530/jrf.0.1170213. J Reprod Fertil. 1999. PMID: 10690188
-
Suppression of the secretion of luteinizing hormone due to isolation/restraint stress in gonadectomised rams and ewes is influenced by sex steroids.J Endocrinol. 1999 Mar;160(3):469-81. doi: 10.1677/joe.0.1600469. J Endocrinol. 1999. PMID: 10076193
-
A simple model of estrous cycle negative and positive feedback regulation of GnRH secretion.Front Neuroendocrinol. 2020 Apr;57:100837. doi: 10.1016/j.yfrne.2020.100837. Epub 2020 Mar 30. Front Neuroendocrinol. 2020. PMID: 32240664 Review.
-
New insights regarding glucocorticoids, stress and gonadotropin suppression.Front Neuroendocrinol. 2006 Jul;27(2):233-45. doi: 10.1016/j.yfrne.2006.03.335. Epub 2006 May 19. Front Neuroendocrinol. 2006. PMID: 16712908 Review.
Cited by
-
Neural and endocrine mechanisms underlying stress-induced suppression of pulsatile LH secretion.Mol Cell Endocrinol. 2019 Dec 1;498:110579. doi: 10.1016/j.mce.2019.110579. Epub 2019 Sep 12. Mol Cell Endocrinol. 2019. PMID: 31521706 Free PMC article. Review.
-
Peripheral Inhibitor of AChE, Neostigmine, Prevents the Inflammatory Dependent Suppression of GnRH/LH Secretion during the Follicular Phase of the Estrous Cycle.Biomed Res Int. 2017;2017:6823209. doi: 10.1155/2017/6823209. Epub 2017 Aug 15. Biomed Res Int. 2017. PMID: 28894751 Free PMC article.
-
Norepinephrine Neurons in the Nucleus of the Solitary Tract Suppress Luteinizing Hormone Secretion in Female Mice.J Neurosci. 2024 Aug 21;44(34):e0084242024. doi: 10.1523/JNEUROSCI.0084-24.2024. J Neurosci. 2024. PMID: 39038954 Free PMC article.
-
Regulation of GnRH pulsatility in ewes.Reproduction. 2018 Sep;156(3):R83-R99. doi: 10.1530/REP-18-0127. Epub 2018 Jun 7. Reproduction. 2018. PMID: 29880718 Free PMC article. Review.
-
A CRH Receptor Type 1 Agonist Increases GABA Transmission to GnRH Neurons in a Circulating-Estradiol-Dependent Manner.Endocrinology. 2020 Nov 1;161(11):bqaa140. doi: 10.1210/endocr/bqaa140. Endocrinology. 2020. PMID: 32798220 Free PMC article.
References
-
- Breen KM, Karsch FJ 2006 New insights regarding glucocorticoids, stress and gonadotropin suppression. Front Neuroendocrinol 27:233–245 - PubMed
-
- Tilbrook AJ, Turner AI, Clarke IJ 2000 Effects of stress on reproduction in non-rodent mammals: the role of glucocorticoids and sex differences. Rev Reprod 5:105–113 - PubMed
-
- Dobson H, Smith RF 2000 What is stress, and how does it affect reproduction? Anim Reprod Sci 60–61:743–752 - PubMed
-
- Rivier C, Rivest S 1991 Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms. Biol Reprod 45:523–532 - PubMed
-
- Ferin M 1993 Neuropeptides, the stress response, and the hypothalamo-pituitary-gonadal axis in the female rhesus monkey. Ann NY Acad Sci 697:106–116 - PubMed

