Resolution of allergic inflammation and airway hyperreactivity is dependent upon disruption of the T1/ST2-IL-33 pathway
- PMID: 19179489
- PMCID: PMC2675564
- DOI: 10.1164/rccm.200805-666OC
Resolution of allergic inflammation and airway hyperreactivity is dependent upon disruption of the T1/ST2-IL-33 pathway
Abstract
Rationale: Although there have been numerous studies on the development of allergen-induced inflammation, the mechanisms leading to resolution of inflammation remain poorly understood. This represents an important consideration because failure to resolve allergen driven inflammation potentially leads to irreversible airway remodeling, characteristic of chronic asthma.
Objectives: We investigated the resolution of allergic inflammation and identified the factors responsible.
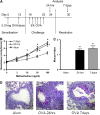
Methods: BALB/c and C57BL/6 mice were sensitized to ovalbumin and challenged through the airways to induce allergic inflammation. Mice were analyzed at 24 hours and 7 days after the final challenge.
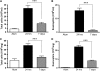
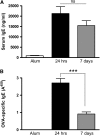
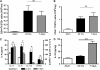
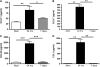
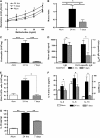
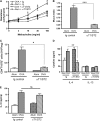
Measurements and main results: Airway hyperreactivity (AHR) and increased mucus production were present 7 days after the cessation of allergen challenge in BALB/c mice. Persisting AHR correlated with the continued presence of Th2 cells but not eosinophils in the lungs. The role of Th2 cells in maintaining AHR was confirmed using blocking antibodies against T1/ST2, IL-4, and IL-13 during the resolution period. Moreover, AHR in the "Th1 type" C57BL/6 mouse strain was resolved 1 week after allergen challenge, concomitant with clearance of Th2 cells from the lung. Expression of the T1/ST2 ligand, IL-33, also correlated with maintenance of AHR.
Conclusions: We have used blockade of Th2 function and strain differences to show for the first time that resolution of allergic inflammation and AHR may be dependent on the T1/ST2-IL-33 pathway and the presence of Th2 cells, suggesting they are necessary not only for the development of an allergic response but also for its maintenance.
Figures

References
-
- Lloyd CM, Gonzalo JA, Coyle AJ, Gutierrez-Ramos JC. Mouse models of allergic airway disease. Adv Immunol 2001;77:263–295. - PubMed
-
- Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma: from bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 2000;161:1720–1745. - PubMed
-
- Kay AB. The role of eosinophils in the pathogenesis of asthma. Trends Mol Med 2005;11:148–152. - PubMed
-
- Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna E, Ghiran S, Gerard NP, Yu C, Orkin SH, et al. A critical role for eosinophils in allergic airways remodeling. Science 2004;305:1776–1779. - PubMed
-
- Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science 2004;305:1773–1776. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

