Very high aquaporin-1 facilitated water permeability in mouse gallbladder
- PMID: 19179619
- PMCID: PMC2670675
- DOI: 10.1152/ajpgi.90680.2008
Very high aquaporin-1 facilitated water permeability in mouse gallbladder
Abstract
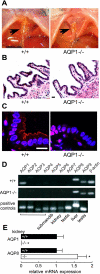
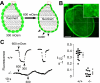
Water transport across gallbladder epithelium is driven by osmotic gradients generated from active salt absorption and secretion. Aquaporin (AQP) water channels have been proposed to facilitate transepithelial water transport in gallbladder and to modulate bile composition. We found strong AQP1 immunofluorescence at the apical membrane of mouse gallbladder epithelium. Transepithelial osmotic water permeability (Pf) was measured in freshly isolated gallbladder sacs from the kinetics of luminal calcein self-quenching in response to an osmotic gradient. Pf was very high (0.12 cm/s) in gallbladders from wild-type mice, cAMP independent, and independent of osmotic gradient size and direction. Although gallbladders from AQP1 knockout mice had similar size and morphology to those from wild-type mice, their Pf was reduced by approximately 10-fold. Apical plasma membrane water permeability was greatly reduced in AQP1-deficient gallbladders, as measured by cytoplasmic calcein quenching in perfluorocarbon-filled, inverted gallbladder sacs. However, neither bile osmolality nor bile salt concentration differed in gallbladders from wild-type vs. AQP1 knockout mice. Our data indicate constitutively high water permeability in mouse gallbladder epithelium involving transcellular water transport through AQP1. The similar bile salt concentration in gallbladders from AQP1 knockout mice argues against a physiologically important role for AQP1 in mouse gallbladder.
Figures


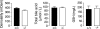
Similar articles
-
Osmotic water permeability diversification in primary trophoblast cultures from aquaporin 1-deficient pregnant mice.J Obstet Gynaecol Res. 2015 Sep;41(9):1399-405. doi: 10.1111/jog.12737. Epub 2015 May 25. J Obstet Gynaecol Res. 2015. PMID: 26014508
-
Expression and subcellular localization of the AQP8 and AQP1 water channels in the mouse gall-bladder epithelium.Biol Cell. 2005 Jun;97(6):415-23. doi: 10.1042/BC20040137. Biol Cell. 2005. PMID: 15859952
-
Unimpaired osmotic water permeability and fluid secretion in bile duct epithelia of AQP1 null mice.Am J Physiol Gastrointest Liver Physiol. 2002 Sep;283(3):G739-46. doi: 10.1152/ajpgi.00540.2001. Am J Physiol Gastrointest Liver Physiol. 2002. PMID: 12181190
-
Aquaporin-1 in the peritoneal membrane: Implications for water transport across capillaries and peritoneal dialysis.Biochim Biophys Acta. 2006 Aug;1758(8):1078-84. doi: 10.1016/j.bbamem.2006.02.025. Epub 2006 Mar 20. Biochim Biophys Acta. 2006. PMID: 16581016 Review.
-
Aquaporins in Urinary System.Adv Exp Med Biol. 2017;969:131-148. doi: 10.1007/978-94-024-1057-0_9. Adv Exp Med Biol. 2017. PMID: 28258571 Review.
Cited by
-
Cholesterol gallstone disease: focusing on the role of gallbladder.Lab Invest. 2015 Feb;95(2):124-31. doi: 10.1038/labinvest.2014.140. Epub 2014 Dec 15. Lab Invest. 2015. PMID: 25502177 Review.
-
Retrospective analysis of canine gallbladder contents in biliary sludge and gallbladder mucoceles.J Vet Med Sci. 2017 Feb 28;79(2):366-374. doi: 10.1292/jvms.16-0562. Epub 2016 Dec 17. J Vet Med Sci. 2017. PMID: 27990011 Free PMC article.
-
Aquaporin-1 Deficiency Protects Against Myocardial Infarction by Reducing Both Edema and Apoptosis in Mice.Sci Rep. 2015 Sep 8;5:13807. doi: 10.1038/srep13807. Sci Rep. 2015. PMID: 26348407 Free PMC article.
-
Aquaporins in Glandular Secretion.Adv Exp Med Biol. 2023;1398:225-249. doi: 10.1007/978-981-19-7415-1_16. Adv Exp Med Biol. 2023. PMID: 36717498
-
Physiological and pathological impact of AQP1 knockout in mice.Biosci Rep. 2019 May 14;39(5):BSR20182303. doi: 10.1042/BSR20182303. Print 2019 May 31. Biosci Rep. 2019. PMID: 31023968 Free PMC article. Review.
References
-
- Calamita G, Ferri D, Bazzini C, Mazzone A, Bottà G, Liquori GE, Paulmichl M, Portincasa P, Meyer G, Svelto M. Expression and subcellular localization of the AQP8 and AQP1 water channels in the mouse gall-bladder epithelium. Biol Cell 97: 415–423, 2005. - PubMed
-
- Diamond JM Osmotic water flow in leaky epithelia. J Membr Biol 51: 195–216, 1979. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

