Lysophosphatidyl choline modulates mechanosensitive L-type Ca2+ current in circular smooth muscle cells from human jejunum
- PMID: 19179622
- PMCID: PMC2670668
- DOI: 10.1152/ajpgi.90610.2008
Lysophosphatidyl choline modulates mechanosensitive L-type Ca2+ current in circular smooth muscle cells from human jejunum
Abstract
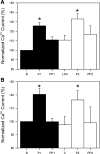
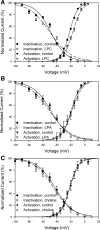
The L-type Ca2+ channel expressed in gastrointestinal smooth muscle is mechanosensitive. Direct membrane stretch and shear stress result in increased Ca2+ entry into the cell. The mechanism for mechanosensitivity is not known, and mechanosensitivity is not dependent on an intact cytoskeleton. The aim of this study was to determine whether L-type Ca2+ channel mechanosensitivity is dependent on tension in the lipid bilayer in human jejunal circular layer myocytes. Whole cell currents were recorded in the amphotericin-perforated-patch configuration, and lysophosphatidyl choline (LPC), lysophosphatidic acid (LPA), and choline were used to alter differentially the tension in the lipid bilayer. Shear stress (perfusion at 10 ml/min) was used to mechanostimulate L-type Ca2+ channels. The increase in L-type Ca2+ current induced by shear stress was greater in the presence of LPC (large head-to-tail proportions), but not LPA or choline, than in the control perfusion. The increased peak Ca2+ current also did not return to baseline levels as in control conditions. Furthermore, steady-state inactivation kinetics were altered in the presence of LPC, leading to a change in window current. These findings suggest that changes in tension in the plasmalemmal membrane can be transmitted to the mechanosensitive L-type Ca2+ channel, leading to altered activity and Ca2+ entry in the human jejunal circular layer myocyte.
Figures




Similar articles
-
A mechanosensitive calcium channel in human intestinal smooth muscle cells.Gastroenterology. 1999 Oct;117(4):900-5. doi: 10.1016/s0016-5085(99)70349-5. Gastroenterology. 1999. PMID: 10500073
-
Whole cell current and membrane potential regulation by a human smooth muscle mechanosensitive calcium channel.Am J Physiol Gastrointest Liver Physiol. 2000 Dec;279(6):G1155-61. doi: 10.1152/ajpgi.2000.279.6.G1155. Am J Physiol Gastrointest Liver Physiol. 2000. PMID: 11093937
-
Fluid flow-induced increase in inward Ba2+ current expressed in HEK293 cells transiently transfected with human neuronal L-type Ca2+ channels.Brain Res. 2005 May 31;1045(1-2):116-23. doi: 10.1016/j.brainres.2005.03.039. Epub 2005 Apr 25. Brain Res. 2005. PMID: 15910769
-
Actin cytoskeletons regulate the stretch-induced increase of Ca2+ current in human gastric myocytes.Biochem Biophys Res Commun. 2007 Jan 12;352(2):503-8. doi: 10.1016/j.bbrc.2006.11.051. Epub 2006 Nov 17. Biochem Biophys Res Commun. 2007. PMID: 17126300
-
alpha(1C) (Ca(V)1.2) L-type calcium channel mediates mechanosensitive calcium regulation.Am J Physiol Cell Physiol. 2002 Sep;283(3):C1001-8. doi: 10.1152/ajpcell.00140.2002. Am J Physiol Cell Physiol. 2002. PMID: 12176756
Cited by
-
Recent advances in small bowel diseases: Part II.World J Gastroenterol. 2012 Jul 14;18(26):3353-74. doi: 10.3748/wjg.v18.i26.3353. World J Gastroenterol. 2012. PMID: 22807605 Free PMC article. Review.
-
Intra-Colonial Functional Differentiation-Related Modulation of the Cellular Membrane in a Pocilloporid Coral Seriatopora caliendrum.Mar Biotechnol (NY). 2015 Oct;17(5):633-43. doi: 10.1007/s10126-015-9645-9. Epub 2015 Aug 5. Mar Biotechnol (NY). 2015. PMID: 26242752
-
Capsaicin as an amphipathic modulator of NaV1.5 mechanosensitivity.Channels (Austin). 2022 Dec;16(1):9-26. doi: 10.1080/19336950.2022.2026015. Channels (Austin). 2022. PMID: 35412435 Free PMC article.
-
Cellular membrane accommodation to thermal oscillations in the coral Seriatopora caliendrum.PLoS One. 2014 Aug 20;9(8):e105345. doi: 10.1371/journal.pone.0105345. eCollection 2014. PLoS One. 2014. PMID: 25140803 Free PMC article.
-
Physiological and pathological functions of mechanosensitive ion channels.Mol Neurobiol. 2014 Oct;50(2):339-47. doi: 10.1007/s12035-014-8654-4. Epub 2014 Feb 15. Mol Neurobiol. 2014. PMID: 24532247 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

