Stimulation of mucosal secretion by lubiprostone (SPI-0211) in guinea pig small intestine and colon
- PMID: 19179625
- PMCID: PMC2670663
- DOI: 10.1152/ajpgi.90447.2008
Stimulation of mucosal secretion by lubiprostone (SPI-0211) in guinea pig small intestine and colon
Abstract
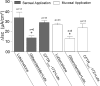
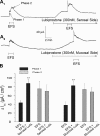
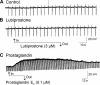
Actions of lubiprostone, a selective type-2 chloride channel activator, on mucosal secretion were investigated in guinea pig small intestine and colon. Flat-sheet preparations were mounted in Ussing flux chambers for recording short-circuit current (Isc) as a marker for electrogenic chloride secretion. Lubiprostone, applied to the small intestinal mucosa in eight concentrations ranging from 1-3000 nM, evoked increases in Isc in a concentration-dependent manner with an EC50 of 42.5 nM. Lubiprostone applied to the mucosa of the colon in eight concentrations ranging from 1-3000 nM evoked increases in Isc in a concentration-dependent manner with an EC50 of 31.7 nM. Blockade of enteric nerves by tetrodotoxin did not influence stimulation of Isc by lubiprostone. Antagonists acting at prostaglandin (PG)E2, EP1-3, or EP4 receptors did not suppress stimulation of Isc by lubiprostone but suppressed or abolished PGE2-evoked responses. Substitution of gluconate for chloride abolished all responses to lubiprostone. The selective CFTR channel blocker, CFTR(inh)-172, did not suppress lubiprostone-evoked Isc. The broadly acting blocker, glibenclamide, suppressed (P<0.001) lubiprostone-evoked Isc. Lubiprostone, in the presence of tetrodotoxin, enhanced carbachol-evoked Isc. The cholinergic component, but not the putative vasoactive intestinal peptide component, of neural responses to electrical field stimulation was enhanced by lubiprostone. Application of any of the prostaglandins, E2, F2, or I2, evoked depolarization of the resting membrane potential in enteric neurons. Unlike the prostaglandins, lubiprostone did not alter the electrical behavior of enteric neurons. Exposure to the histamine H2 receptor agonists increased basal Isc followed by persistent cyclical increases in Isc. Lubiprostone increased the peak amplitude of the dimaprit-evoked cycles.
Figures
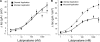
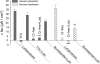






Similar articles
-
Lubiprostone reverses the inhibitory action of morphine on intestinal secretion in guinea pig and mouse.J Pharmacol Exp Ther. 2010 Jul;334(1):333-40. doi: 10.1124/jpet.110.166116. Epub 2010 Apr 20. J Pharmacol Exp Ther. 2010. PMID: 20406855 Free PMC article.
-
Lubiprostone reverses the inhibitory action of morphine on mucosal secretion in human small intestine.Dig Dis Sci. 2011 Feb;56(2):330-8. doi: 10.1007/s10620-010-1515-8. Epub 2010 Dec 23. Dig Dis Sci. 2011. PMID: 21181441 Free PMC article.
-
A multi-tyrosinated sst1/2 receptor preferring somatostatin agonist inhibits reflex and immune-mediated secretion in the guinea pig colon.Regul Pept. 2003 Jun 15;114(1):51-60. doi: 10.1016/s0167-0115(03)00108-3. Regul Pept. 2003. PMID: 12763640
-
Glucagon-like peptide-1 modulates neurally evoked mucosal chloride secretion in guinea pig small intestine in vitro.Am J Physiol Gastrointest Liver Physiol. 2012 Feb 1;302(3):G352-8. doi: 10.1152/ajpgi.00333.2011. Epub 2011 Nov 10. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22075777 Free PMC article.
-
Enteric nervous system: sensory physiology, diarrhea and constipation.Curr Opin Gastroenterol. 2010 Mar;26(2):102-8. doi: 10.1097/MOG.0b013e328334df4f. Curr Opin Gastroenterol. 2010. PMID: 19926984 Review.
Cited by
-
Lubiprostone activates Cl- secretion via cAMP signaling and increases membrane CFTR in the human colon carcinoma cell line, T84.Dig Dis Sci. 2011 Feb;56(2):339-51. doi: 10.1007/s10620-010-1495-8. Epub 2010 Dec 8. Dig Dis Sci. 2011. PMID: 21140215
-
Lubiprostone ameliorates the cystic fibrosis mouse intestinal phenotype.BMC Gastroenterol. 2010 Sep 15;10:107. doi: 10.1186/1471-230X-10-107. BMC Gastroenterol. 2010. PMID: 20843337 Free PMC article.
-
Effects of lubiprostone on pacemaker activity of interstitial cells of cajal from the mouse colon.Korean J Physiol Pharmacol. 2014 Aug;18(4):341-6. doi: 10.4196/kjpp.2014.18.4.341. Epub 2014 Aug 13. Korean J Physiol Pharmacol. 2014. PMID: 25177167 Free PMC article.
-
The ClC-2 Chloride Channel Activator, Lubiprostone, Improves Intestinal Barrier Function in Biopsies from Crohn's Disease but Not Ulcerative Colitis Patients.Pharmaceutics. 2023 Mar 2;15(3):811. doi: 10.3390/pharmaceutics15030811. Pharmaceutics. 2023. PMID: 36986672 Free PMC article.
-
Lubiprostone reverses the inhibitory action of morphine on intestinal secretion in guinea pig and mouse.J Pharmacol Exp Ther. 2010 Jul;334(1):333-40. doi: 10.1124/jpet.110.166116. Epub 2010 Apr 20. J Pharmacol Exp Ther. 2010. PMID: 20406855 Free PMC article.
References
-
- Bassil AK, Thangiah R, Borman RA, Jarvie EM, Vivekanandan S, Lalude O, Sung EZ, Baragwanath P, Wong LS, Nwokolo CU, Lee K, Sanger GJ. Effect of lubiprostone on rat and human colon muscle: possible involvement of prostaglandin EP receptors (Abstract). Gastroenterology 132: A455, M2123, 2007.
-
- Calderaro V, Giovane A, De Simone B, Camussi G, Rossiello R, Quagliuolo L, Servillo L, Taccone W, Giordano C, Balestrieri C. Arachidonic acid metabolites and chloride secretion in rabbit distal colonic mucosa. Am J Physiol Gastrointest Liver Physiol 261: G443–G450, 1991. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

