Learning a prior on regulatory potential from eQTL data
- PMID: 19180192
- PMCID: PMC2627940
- DOI: 10.1371/journal.pgen.1000358
Learning a prior on regulatory potential from eQTL data
Abstract
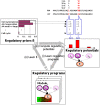
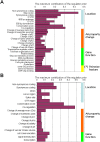
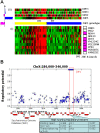
Genome-wide RNA expression data provide a detailed view of an organism's biological state; hence, a dataset measuring expression variation between genetically diverse individuals (eQTL data) may provide important insights into the genetics of complex traits. However, with data from a relatively small number of individuals, it is difficult to distinguish true causal polymorphisms from the large number of possibilities. The problem is particularly challenging in populations with significant linkage disequilibrium, where traits are often linked to large chromosomal regions containing many genes. Here, we present a novel method, Lirnet, that automatically learns a regulatory potential for each sequence polymorphism, estimating how likely it is to have a significant effect on gene expression. This regulatory potential is defined in terms of "regulatory features"-including the function of the gene and the conservation, type, and position of genetic polymorphisms-that are available for any organism. The extent to which the different features influence the regulatory potential is learned automatically, making Lirnet readily applicable to different datasets, organisms, and feature sets. We apply Lirnet both to the human HapMap eQTL dataset and to a yeast eQTL dataset and provide statistical and biological results demonstrating that Lirnet produces significantly better regulatory programs than other recent approaches. We demonstrate in the yeast data that Lirnet can correctly suggest a specific causal sequence variation within a large, linked chromosomal region. In one example, Lirnet uncovered a novel, experimentally validated connection between Puf3-a sequence-specific RNA binding protein-and P-bodies-cytoplasmic structures that regulate translation and RNA stability-as well as the particular causative polymorphism, a SNP in Mkt1, that induces the variation in the pathway.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Yvert G, Brem RB, Whittle J, Akey JM, Foss E, et al. Trans-acting regulatory variation in Saccharomyces cerevisiae and the role of transcription factors. Nat Genet. 2003;35:57–64. - PubMed
-
- Schadt EE, Monks SA, Drake TA, Lusis AJ, Che N, et al. Genetics of gene expression surveyed in maize, mouse and man. Nature. 2003;422:297–302. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

