B cell depletion with rituximab in patients with diffuse cutaneous systemic sclerosis
- PMID: 19180481
- PMCID: PMC2637937
- DOI: 10.1002/art.24249
B cell depletion with rituximab in patients with diffuse cutaneous systemic sclerosis
Abstract
Objective: To determine the safety of rituximab, to provide preliminary data regarding the potential efficacy of rituximab, and to investigate the effects of rituximab on autoimmunity and fibrosis in patients with diffuse cutaneous systemic sclerosis (dcSSc).
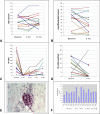
Methods: Fifteen patients with dcSSc, all of whom experienced their first non-Raynaud's disease-associated disease manifestation within 18 months of trial entry, were recruited to receive 2 intravenous doses of rituximab (1,000 mg), administered 2 weeks apart. Safety, clinical, and exploratory outcomes were evaluated at baseline and at 6 months. The primary outcome was the change in the modified Rodnan skin thickness score (MRSS) at 6 months compared with baseline.
Results: Adverse events included frequent infusion reactions and rare infections (urinary tract infection and dental abscess occurred in 1 patient each). The mean change in the MRSS between baseline and 6 months was not significant. Results of pulmonary function tests and other measures of major organ involvement were stable. The modest B cell infiltrates that were present in most skin biopsy specimens at baseline were completely depleted at 6 months in most patients. Autoantibody titers showed only modest and variable changes after treatment.
Conclusion: In this pilot study, treatment with rituximab appeared to be safe and well tolerated among patients with dcSSc. Rituximab treatment resulted in both depletion of circulating B cells and depletion of dermal B cells but had little effect on the levels of SSc-associated autoantibodies. Rituximab treatment did not appear to result in a significant beneficial effect on skin disease. The potential efficacy of rituximab in other organs such as the lung could not be clearly evaluated in this small open-label trial.
Figures
Comment in
-
B-cell-targeted therapy for the fibrotic complications of systemic sclerosis.Curr Rheumatol Rep. 2011 Feb;13(1):1-3. doi: 10.1007/s11926-010-0150-x. Curr Rheumatol Rep. 2011. PMID: 21107765 No abstract available.
References
-
- Baroni SS, Santillo M, Bevilacqua F, Luchetti M, Spadoni T, Mancini M, et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med. 2006;354(25):2667–76. - PubMed
-
- Matsushita T, Fujimoto M, Hasegawa M, Matsushita Y, Komura K, Ogawa F, et al. BAFF antagonist attenuates the development of skin fibrosis in tight-skin mice. J Invest Dermatol. 2007;127(12):2772–80. - PubMed
-
- Lafyatis R, O'Hara C, Feghali-Bostwick CA, Matteson E. B cell infiltration in systemic sclerosis-associated interstitial lung disease. Arthritis Rheum. 2007;56(9):3167–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

