Blind identification of evoked human brain activity with independent component analysis of optical data
- PMID: 19180556
- PMCID: PMC6870678
- DOI: 10.1002/hbm.20678
Blind identification of evoked human brain activity with independent component analysis of optical data
Abstract
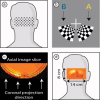
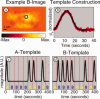
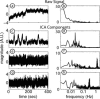
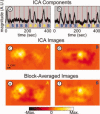
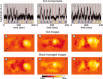
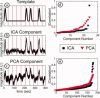
Diffuse optical tomography (DOT) methods observe hemodynamics in the brain by measuring light transmission through the scalp, skull, and brain. Thus, separating signals due to heart pulsations, breathing movements, and systemic blood flow fluctuations from the desired brain functional responses is critical to the fidelity of the derived maps. Herein, we applied independent component analysis (ICA) to temporal signals obtained from a high-density DOT system used for functional mapping of the visual cortex. DOT measurements were taken over the occipital cortex of human adult subjects while they viewed stimuli designed to activate two spatially distinct areas of the visual cortex. ICA was able to extract clean functional hemodynamic signals and separate brain activity sources from hemodynamic fluctuations related to heart and breathing without knowledge of the stimulus paradigm. Furthermore, independent components were found defining distinct functional responses to each stimulus type. Images generated from single ICA components were comparable, with regard to spatial extent and resolution, to images from block averaging (with knowledge of the block stimulus paradigm). Both images and estimated time-series signals demonstrated that ICA was superior to principal component analysis in extracting the true event-evoked response signals. Our results suggest that ICA can extract the time courses and the corresponding spatial extent of functional responses in DOT imaging.
(c) 2009 Wiley-Liss, Inc.
Figures

References
-
- Bartels A,Zeki S ( 2004): The chronoarchitecture of the human brain—Natural viewing conditions reveal a time‐based anatomy of the brain. NeuroImage 22: 419–433. - PubMed
-
- Bartels A,Zeki S ( 2005): Brain dynamics during natural viewing conditions–A new guide for mapping connectivity in vivo. NeuroImage 24: 339–349. - PubMed
-
- Bell AJ,Sejnowski TJ ( 1995): An information‐maximization approach to blind separation and blind deconvolution. Neural Comput 7: 1129–1159. - PubMed
-
- Belouchrani A,Abed‐Meraim K,Cardoso JF,Moulines D ( 1997): A blind source separation technique using second order statistics. IEEE Trans Signal Process 45: 434–444.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

