Shape-selective interception by hydrocarbons of the O2-derived oxidant of a biomimetic nonheme iron complex
- PMID: 19180613
- PMCID: PMC2719302
- DOI: 10.1002/anie.200805342
Shape-selective interception by hydrocarbons of the O2-derived oxidant of a biomimetic nonheme iron complex
Abstract
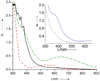
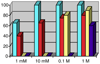
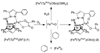
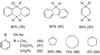
Picky ferryl: The complex [Fe(Tp(Ph(2)))(BF)] (Tp(Ph(2)) = hydrotris(3,5-diphenylpyrazolyl)borate; BF = benzoylformate) reacts with O(2) to generate an oxidant (see picture; O red, pink; Fe yellow; N blue; C gray; H white) that oxidizes added hydrocarbons shape-selectively. Discrimination derives from a cleft formed by two phenyl groups of the Tp(Ph(2)) ligand, favoring oblate spheroidal substrates.
Figures

Comment in
-
Biomimetic trispyrazolylborato iron complexes.Angew Chem Int Ed Engl. 2009;48(31):5580-2. doi: 10.1002/anie.200900552. Angew Chem Int Ed Engl. 2009. PMID: 19504506 No abstract available.
Similar articles
-
Aerobic alcohol oxidation and oxygen atom transfer reactions catalyzed by a nonheme iron(ii)-α-keto acid complex.Chem Sci. 2016 Aug 1;7(8):5322-5331. doi: 10.1039/c6sc01476c. Epub 2016 Apr 25. Chem Sci. 2016. PMID: 30155184 Free PMC article.
-
Reactivity of an iron-oxygen oxidant generated upon oxidative decarboxylation of biomimetic iron(II) α-hydroxy acid complexes.Inorg Chem. 2014 Mar 17;53(6):2810-21. doi: 10.1021/ic402443r. Epub 2014 Mar 1. Inorg Chem. 2014. PMID: 24627956
-
Bioinspired Nonheme Iron Catalysts for C-H and C═C Bond Oxidation: Insights into the Nature of the Metal-Based Oxidants.Acc Chem Res. 2015 Sep 15;48(9):2612-21. doi: 10.1021/acs.accounts.5b00053. Epub 2015 Aug 17. Acc Chem Res. 2015. PMID: 26280131
-
Bio-inspired Nonheme Iron Oxidation Catalysis: Involvement of Oxoiron(V) Oxidants in Cleaving Strong C-H Bonds.Angew Chem Int Ed Engl. 2020 May 4;59(19):7332-7349. doi: 10.1002/anie.201906551. Epub 2020 Mar 2. Angew Chem Int Ed Engl. 2020. PMID: 31373120 Review.
-
High-valent nonheme iron-oxo species in biomimetic oxidations.J Inorg Biochem. 2006 Apr;100(4):421-33. doi: 10.1016/j.jinorgbio.2006.01.014. Epub 2006 Mar 13. J Inorg Biochem. 2006. PMID: 16530841 Review.
Cited by
-
Oxygen activation at mononuclear nonheme iron centers: a superoxo perspective.Inorg Chem. 2010 Apr 19;49(8):3618-28. doi: 10.1021/ic901891n. Inorg Chem. 2010. PMID: 20380464 Free PMC article.
-
Functional models of α-keto acid dependent nonheme iron oxygenases: synthesis and reactivity of biomimetic iron(II) benzoylformate complexes supported by a 2,9-dimethyl-1,10-phenanthroline ligand.J Biol Inorg Chem. 2013 Mar;18(3):401-10. doi: 10.1007/s00775-013-0984-6. Epub 2013 Feb 16. J Biol Inorg Chem. 2013. PMID: 23417539
-
Intramolecular gas-phase reactions of synthetic nonheme oxoiron(IV) ions: proximity and spin-state reactivity rules.Chemistry. 2012 Sep 10;18(37):11747-60. doi: 10.1002/chem.201200105. Epub 2012 Jul 26. Chemistry. 2012. PMID: 22837063 Free PMC article.
-
Mangana(iii/iv)electro-catalyzed C(sp3)-H azidation.Chem Sci. 2020 Dec 28;12(8):2890-2897. doi: 10.1039/d0sc05924b. Chem Sci. 2020. PMID: 34164055 Free PMC article.
-
Aerobic alcohol oxidation and oxygen atom transfer reactions catalyzed by a nonheme iron(ii)-α-keto acid complex.Chem Sci. 2016 Aug 1;7(8):5322-5331. doi: 10.1039/c6sc01476c. Epub 2016 Apr 25. Chem Sci. 2016. PMID: 30155184 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

