Fat synthesis and adiposity regulation in Caenorhabditis elegans
- PMID: 19181539
- PMCID: PMC2665873
- DOI: 10.1016/j.tem.2008.11.002
Fat synthesis and adiposity regulation in Caenorhabditis elegans
Abstract
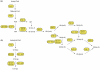
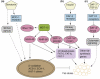
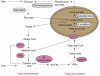
Understanding the regulation of fat synthesis and the consequences of its misregulation is of profound significance for managing the obesity epidemic and developing obesity therapeutics. Recent work in the roundworm Caenorhabditis elegans has revealed the importance of evolutionarily conserved pathways of fat synthesis and nutrient sensing in adiposity regulation. The powerful combination of mutational and reverse genetic analysis, genomics, lipid analysis, and cell-specific expression studies enables dissection of complicated pathways at the level of a whole organism. This review summarizes recent studies in C. elegans that offer insights into the regulation of adiposity by conserved transcription factors, insulin and growth factor signaling, and unsaturated fatty acid synthesis. Increased understanding of fat-storage pathways might lead to future obesity therapies.
Figures

References
-
- Lawrence VJ, Kopelman PG. Medical consequences of obesity. Clin. Dermatol. 2004;22:296–302. - PubMed
-
- Badman MK, Flier JS. The adipocyte as an active participant in energy balance and metabolism. Gastroenterology. 2007;132:2103–2115. - PubMed
-
- Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–350. - PubMed
-
- Herbert A, et al. A common genetic variant is associated with adult and childhood obesity. Science. 2006;312:279–283. - PubMed
-
- Stanley S, et al. Hormonal regulation of food intake. Physiol. Rev. 2005;85:1131–1158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

