Codon-optimized fluorescent proteins designed for expression in low-GC gram-positive bacteria
- PMID: 19181829
- PMCID: PMC2663184
- DOI: 10.1128/AEM.02066-08
Codon-optimized fluorescent proteins designed for expression in low-GC gram-positive bacteria
Abstract
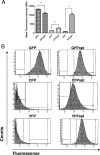
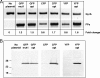
Fluorescent proteins have wide applications in biology. However, not all of these proteins are properly expressed in bacteria, especially if the codon usage and genomic GC content of the host organism are not ideal for high expression. In this study, we analyzed the DNA sequences of multiple fluorescent protein genes with respect to codons and GC content and compared them to a low-GC gram-positive bacterium, Bacillus anthracis. We found high discrepancies for cyan fluorescent protein (CFP), yellow fluorescent protein (YFP), and the photoactivatable green fluorescent protein (PAGFP), but not GFP, with regard to GC content and codon usage. Concomitantly, when the proteins were expressed in B. anthracis, CFP- and YFP-derived fluorescence was undetectable microscopically, a phenomenon caused not by lack of gene transcription or degradation of the proteins but by lack of protein expression. To improve expression in bacteria with low genomic GC contents, we synthesized a codon-optimized gfp and constructed optimized photoactivatable pagfp, cfp, and yfp, which were in contrast to nonoptimized genes highly expressed in B. anthracis and in another low-GC gram-positive bacterium, Staphylococcus aureus. Using optimized GFP as a reporter, we were able to monitor the activity of the protective antigen promoter of B. anthracis and confirm its dependence on bicarbonate and regulators present on virulence plasmid pXO1.
Figures



Similar articles
-
Genetic tools to enhance the study of gene function and regulation in Staphylococcus aureus.Appl Environ Microbiol. 2013 Apr;79(7):2218-24. doi: 10.1128/AEM.00136-13. Epub 2013 Jan 25. Appl Environ Microbiol. 2013. PMID: 23354696 Free PMC article.
-
Fluorescent reporters for studies of cellular localization of proteins in Staphylococcus aureus.Appl Environ Microbiol. 2010 Jul;76(13):4346-53. doi: 10.1128/AEM.00359-10. Epub 2010 May 7. Appl Environ Microbiol. 2010. PMID: 20453129 Free PMC article.
-
Yeast-enhanced green fluorescent protein (yEGFP): a reporter of gene expression in Candida albicans.Microbiology (Reading). 1997 Feb;143 ( Pt 2):303-311. doi: 10.1099/00221287-143-2-303. Microbiology (Reading). 1997. PMID: 9043107
-
Optimization of fluorescent tools for cell biology studies in Gram-positive bacteria.PLoS One. 2014 Dec 2;9(12):e113796. doi: 10.1371/journal.pone.0113796. eCollection 2014. PLoS One. 2014. PMID: 25464377 Free PMC article.
-
Bacillus anthracis genetics and virulence gene regulation.Curr Top Microbiol Immunol. 2002;271:143-64. doi: 10.1007/978-3-662-05767-4_7. Curr Top Microbiol Immunol. 2002. PMID: 12224521 Review.
Cited by
-
Distinct roles of phenol-soluble modulins in spreading of Staphylococcus aureus on wet surfaces.Appl Environ Microbiol. 2013 Feb;79(3):886-95. doi: 10.1128/AEM.03157-12. Epub 2012 Nov 26. Appl Environ Microbiol. 2013. PMID: 23183971 Free PMC article.
-
Effect of Substance P in Staphylococcus aureus and Staphylococcus epidermidis Virulence: Implication for Skin Homeostasis.Front Microbiol. 2016 Apr 15;7:506. doi: 10.3389/fmicb.2016.00506. eCollection 2016. Front Microbiol. 2016. PMID: 27148195 Free PMC article.
-
Anaerobic Fungal Mevalonate Pathway Genomic Biases Lead to Heterologous Toxicity Underpredicted by Codon Adaptation Indices.Microorganisms. 2021 Sep 18;9(9):1986. doi: 10.3390/microorganisms9091986. Microorganisms. 2021. PMID: 34576881 Free PMC article.
-
Transcriptional Analysis and Subcellular Protein Localization Reveal Specific Features of the Essential WalKR System in Staphylococcus aureus.PLoS One. 2016 Mar 21;11(3):e0151449. doi: 10.1371/journal.pone.0151449. eCollection 2016. PLoS One. 2016. PMID: 26999783 Free PMC article.
-
Bacillus anthracis Virulence Regulator AtxA Binds Specifically to the pagA Promoter Region.J Bacteriol. 2019 Nov 5;201(23):e00569-19. doi: 10.1128/JB.00569-19. Print 2019 Dec 1. J Bacteriol. 2019. PMID: 31570528 Free PMC article.
References
-
- Beyenal, H., C. Yakymyshyn, J. Hyungnak, C. C. Davis, and Z. Lewandowski. 2004. An optical microsensor to measure fluorescent light intensity in biofilms. J. Microbiol. Methods 58:367-374. - PubMed
-
- Blokpoel, M. C., R. O'Toole, M. J. Smeulders, and H. D. Williams. 2003. Development and application of unstable GFP variants to kinetic studies of mycobacterial gene expression. J. Microbiol. Methods 54:203-211. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

