Improved fermentation performance of a lager yeast after repair of its AGT1 maltose and maltotriose transporter genes
- PMID: 19181838
- PMCID: PMC2675207
- DOI: 10.1128/AEM.01558-08
Improved fermentation performance of a lager yeast after repair of its AGT1 maltose and maltotriose transporter genes
Abstract
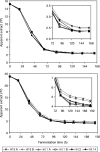
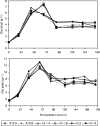
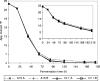
The use of more concentrated, so-called high-gravity and very-high-gravity (VHG) brewer's worts for the manufacture of beer has economic and environmental advantages. However, many current strains of brewer's yeasts ferment VHG worts slowly and incompletely, leaving undesirably large amounts of maltose and especially maltotriose in the final beers. alpha-Glucosides are transported into Saccharomyces yeasts by several transporters, including Agt1, which is a good carrier of both maltose and maltotriose. The AGT1 genes of brewer's ale yeast strains encode functional transporters, but the AGT1 genes of the lager strains studied contain a premature stop codon and do not encode functional transporters. In the present work, one or more copies of the AGT1 gene of a lager strain were repaired with DNA sequence from an ale strain and put under the control of a constitutive promoter. Compared to the untransformed strain, the transformants with repaired AGT1 had higher maltose transport activity, especially after growth on glucose (which represses endogenous alpha-glucoside transporter genes) and higher ratios of maltotriose transport activity to maltose transport activity. They fermented VHG (24 degrees Plato) wort faster and more completely, producing beers containing more ethanol and less residual maltose and maltotriose. The growth and sedimentation behaviors of the transformants were similar to those of the untransformed strain, as were the profiles of yeast-derived volatile aroma compounds in the beers.
Figures



Similar articles
-
Identification of regulatory elements in the AGT1 promoter of ale and lager strains of brewer's yeast.Yeast. 2011 Aug;28(8):579-94. doi: 10.1002/yea.1888. Epub 2011 Jul 13. Yeast. 2011. PMID: 21755532
-
The temperature dependence of maltose transport in ale and lager strains of brewer's yeast.FEMS Yeast Res. 2010 Jun;10(4):402-11. doi: 10.1111/j.1567-1364.2010.00627.x. Epub 2010 Mar 11. FEMS Yeast Res. 2010. PMID: 20402791 Free PMC article.
-
Expression patterns of Mal genes and association with differential maltose and maltotriose transport rate of two Saccharomyces pastorianus yeasts.Appl Environ Microbiol. 2024 Jul 24;90(7):e0039724. doi: 10.1128/aem.00397-24. Epub 2024 Jul 8. Appl Environ Microbiol. 2024. PMID: 38975758 Free PMC article.
-
Maltose and Maltotriose Transporters in Brewer's Saccharomyces Yeasts: Polymorphic and Key Residues in Their Activity.Int J Mol Sci. 2025 Jun 20;26(13):5943. doi: 10.3390/ijms26135943. Int J Mol Sci. 2025. PMID: 40649723 Free PMC article. Review.
-
Saccharomyces pastorianus: genomic insights inspiring innovation for industry.Yeast. 2015 Jan;32(1):17-27. doi: 10.1002/yea.3033. Epub 2014 Sep 23. Yeast. 2015. PMID: 25088523 Review.
Cited by
-
Identification of European isolates of the lager yeast parent Saccharomyces eubayanus.FEMS Yeast Res. 2022 Dec 7;22(1):foac053. doi: 10.1093/femsyr/foac053. FEMS Yeast Res. 2022. PMID: 36473696 Free PMC article.
-
Lager-brewing yeasts in the era of modern genetics.FEMS Yeast Res. 2019 Nov 1;19(7):foz063. doi: 10.1093/femsyr/foz063. FEMS Yeast Res. 2019. PMID: 31553794 Free PMC article. Review.
-
Improved Sugarcane-Based Fermentation Processes by an Industrial Fuel-Ethanol Yeast Strain.J Fungi (Basel). 2023 Jul 29;9(8):803. doi: 10.3390/jof9080803. J Fungi (Basel). 2023. PMID: 37623574 Free PMC article.
-
Laboratory Evolution of a Saccharomyces cerevisiae × S. eubayanus Hybrid Under Simulated Lager-Brewing Conditions.Front Genet. 2019 Mar 29;10:242. doi: 10.3389/fgene.2019.00242. eCollection 2019. Front Genet. 2019. PMID: 31001314 Free PMC article.
-
New lager yeast strains generated by interspecific hybridization.J Ind Microbiol Biotechnol. 2015 May;42(5):769-78. doi: 10.1007/s10295-015-1597-6. Epub 2015 Feb 15. J Ind Microbiol Biotechnol. 2015. PMID: 25682107 Free PMC article.
References
-
- Alves, S. L., R. A. Herberts, C. Hollatz, D. Trichez, L. C. Miletti, P. S. de Araujo, and B. U. Stambuk. 2008. Molecular analysis of maltotriose active transport and fermentation by Saccharomyces cerevisiae reveals a determinant role for the AGT1 permease. Appl. Environ. Microbiol. 74:1494-1501. - PMC - PubMed
-
- Bisson, L. F., D. M. Coons, A. L. Kruckeberg, and D. A. Lewis. 1993. Yeast sugar transporters. Crit. Rev. Biochem. Mol. Biol. 28:259-308. - PubMed
-
- Day, R. E., V. J. Higgins, P. J. Rogers, and I. W. Dawes. 2002. Characterization of the putative maltose transporters encoded by YDL247w and YJR160c. Yeast 19:1015-1027. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

