Detergent binding explains anomalous SDS-PAGE migration of membrane proteins
- PMID: 19181854
- PMCID: PMC2644111
- DOI: 10.1073/pnas.0813167106
Detergent binding explains anomalous SDS-PAGE migration of membrane proteins
Abstract
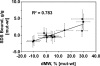
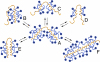
Migration on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) that does not correlate with formula molecular weights, termed "gel shifting," appears to be common for membrane proteins but has yet to be conclusively explained. In the present work, we investigate the anomalous gel mobility of helical membrane proteins using a library of wild-type and mutant helix-loop-helix ("hairpin") sequences derived from transmembrane segments 3 and 4 of the human cystic fibrosis transmembrane conductance regulator (CFTR), including disease-phenotypic residue substitutions. We find that these hairpins migrate at rates of -10% to +30% vs. their actual formula weights on SDS-PAGE and load detergent at ratios ranging from 3.4-10 g SDS/g protein. We additionally demonstrate that mutant gel shifts strongly correlate with changes in hairpin SDS loading capacity (R(2) = 0.8), and with hairpin helicity (R(2) = 0.9), indicating that gel shift behavior originates in altered detergent binding. In some cases, this differential solvation by SDS may result from replacing protein-detergent contacts with protein-protein contacts, implying that detergent binding and folding are intimately linked. The CF-phenotypic V232D mutant included in our library may thus disrupt CFTR function via altered protein-lipid interactions. The observed interdependence between hairpin migration, SDS aggregation number, and conformation additionally suggests that detergent binding may provide a rapid and economical screen for identifying membrane proteins with robust tertiary and/or quaternary structures.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Structural basis for misfolding at a disease phenotypic position in CFTR: comparison of TM3/4 helix-loop-helix constructs with TM4 peptides.Biochim Biophys Acta. 2012 Jan;1818(1):49-54. doi: 10.1016/j.bbamem.2011.09.027. Epub 2011 Oct 3. Biochim Biophys Acta. 2012. PMID: 21996038
-
Role of the extracellular loop in the folding of a CFTR transmembrane helical hairpin.Biochemistry. 2007 Jun 19;46(24):7099-106. doi: 10.1021/bi602570u. Epub 2007 May 22. Biochemistry. 2007. PMID: 17516627
-
Positional dependence of non-native polar mutations on folding of CFTR helical hairpins.Biochim Biophys Acta. 2008 Jan;1778(1):79-87. doi: 10.1016/j.bbamem.2007.08.036. Epub 2007 Sep 15. Biochim Biophys Acta. 2008. PMID: 17949679
-
Two-dimensional electrophoresis of membrane proteins.Anal Bioanal Chem. 2007 Oct;389(4):1033-45. doi: 10.1007/s00216-007-1514-6. Epub 2007 Aug 7. Anal Bioanal Chem. 2007. PMID: 17680235 Review.
-
The cystic fibrosis transmembrane conductance regulator (CFTR) and its stability.Cell Mol Life Sci. 2017 Jan;74(1):23-38. doi: 10.1007/s00018-016-2386-8. Epub 2016 Oct 12. Cell Mol Life Sci. 2017. PMID: 27734094 Free PMC article. Review.
Cited by
-
Ablation of neuronal ceramide synthase 1 in mice decreases ganglioside levels and expression of myelin-associated glycoprotein in oligodendrocytes.J Biol Chem. 2012 Dec 7;287(50):41888-902. doi: 10.1074/jbc.M112.413500. Epub 2012 Oct 16. J Biol Chem. 2012. PMID: 23074226 Free PMC article.
-
Bak apoptotic function is not directly regulated by phosphorylation.Cell Death Dis. 2013 Jan 10;4(1):e452. doi: 10.1038/cddis.2012.191. Cell Death Dis. 2013. PMID: 23303126 Free PMC article.
-
Screening for transmembrane association in divisome proteins using TOXGREEN, a high-throughput variant of the TOXCAT assay.Biochim Biophys Acta. 2016 Nov;1858(11):2573-2583. doi: 10.1016/j.bbamem.2016.07.008. Epub 2016 Jul 22. Biochim Biophys Acta. 2016. PMID: 27453198 Free PMC article.
-
Adiponectin receptor 1 C-terminus interacts with PDZ-domain proteins such as syntrophins.Exp Mol Pathol. 2013 Oct;95(2):180-6. doi: 10.1016/j.yexmp.2013.07.002. Epub 2013 Jul 13. Exp Mol Pathol. 2013. PMID: 23860432 Free PMC article.
-
Roles of the Rabies Virus Phosphoprotein Isoforms in Pathogenesis.J Virol. 2016 Aug 26;90(18):8226-37. doi: 10.1128/JVI.00809-16. Print 2016 Sep 15. J Virol. 2016. PMID: 27384657 Free PMC article.
References
-
- Imamura T. In: Encyclopedia of Surface and Colloid Science. Somasundaran P, editor. New York: Taylor & Francis; 2006. pp. 5251–5263.
-
- Shirahama K, Tsujii K, Takagi T. Free-boundary electrophoresis of sodium dodecyl sulfate-protein polypeptide complexes with special reference to SDS-polyacrylamide gel electrophoresis. J Biochem (Tokyo) 1974;75:309–319. - PubMed
-
- Ibel K, et al. Protein-decorated micelle structure of sodium-dodecyl-sulfate–protein complexes as determined by neutron scattering. Eur J Biochem. 1990;190:311–318. - PubMed
-
- Tanford C. The hydrophobic effect: Formation of micelle and biological membranes. 2nd Ed. New York: Wiley; 1980.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

