Tissue plasminogen activator modulates the cellular and behavioral response to cocaine
- PMID: 19181855
- PMCID: PMC2644150
- DOI: 10.1073/pnas.0812491106
Tissue plasminogen activator modulates the cellular and behavioral response to cocaine
Abstract
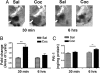
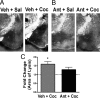
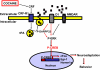
Cocaine exposure induces long-lasting molecular and structural adaptations in the brain. In this study, we show that tissue plasminogen activator (tPA), an extracellular protease involved in neuronal plasticity, modulates the biochemical and behavioral response to cocaine. When injected in the acute binge paradigm, cocaine enhanced tPA activity in the amygdala, which required activation of corticotropin-releasing factor type-1 (CRF-R1) receptors. Compared with WT mice, tPA-/- mice injected with cocaine displayed attenuated phosphorylation of ERK, cAMP response element binding protein (CREB), and dopamine and cAMP-regulated phosphoprotein 32 kDa (DARPP-32) and blunted induction of immediate early genes (IEGs) c-Fos, Egr-1, and Homer 1a in the amygdala and the nucleus accumbens (NAc). tPA-/- mice also displayed significantly higher basal preprodynorphin (ppDyn) mRNA levels in the NAc in comparison to WT mice, and cocaine decreased ppDyn mRNA levels in tPA-/- mice only. Cocaine-induced locomotor sensitization and conditioned place preference (CPP) were attenuated in tPA-/- mice. Cocaine exposure also had an anxiolytic effect in tPA-/- but not WT mice. These results identify tPA as an important and novel component of the signaling pathway that modulates cocaine-induced changes in neuroadaptation and behavior.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

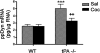


Similar articles
-
Involvement of tissue plasminogen activator in stress responsivity during acute cocaine withdrawal in mice.Stress. 2010 Nov;13(6):481-90. doi: 10.3109/10253891003786415. Epub 2010 Jul 28. Stress. 2010. PMID: 20666641 Free PMC article.
-
Cocaine-Dependent Acquisition of Locomotor Sensitization and Conditioned Place Preference Requires D1 Dopaminergic Signaling through a Cyclic AMP, NCS-Rapgef2, ERK, and Egr-1/Zif268 Pathway.J Neurosci. 2021 Jan 27;41(4):711-725. doi: 10.1523/JNEUROSCI.1497-20.2020. Epub 2020 Dec 2. J Neurosci. 2021. PMID: 33268547 Free PMC article.
-
Tissue plasminogen activator promotes the effects of corticotropin-releasing factor on the amygdala and anxiety-like behavior.Proc Natl Acad Sci U S A. 2004 Nov 16;101(46):16345-50. doi: 10.1073/pnas.0407355101. Epub 2004 Nov 2. Proc Natl Acad Sci U S A. 2004. PMID: 15522965 Free PMC article.
-
[Role of tissue plasminogen activator in the rewarding effect of morphine].Nihon Arukoru Yakubutsu Igakkai Zasshi. 2006 Feb;41(1):23-30. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2006. PMID: 16619846 Review. Japanese.
-
[Regulation by tissue plasminogen activator of rewarding effects of drugs of abuse].Nihon Shinkei Seishin Yakurigaku Zasshi. 2008 Feb;28(1):1-6. Nihon Shinkei Seishin Yakurigaku Zasshi. 2008. PMID: 18411702 Review. Japanese.
Cited by
-
Matrix metalloproteinases, synaptic injury, and multiple sclerosis.Front Psychiatry. 2010 Oct 5;1:130. doi: 10.3389/fpsyt.2010.00130. eCollection 2010. Front Psychiatry. 2010. PMID: 21423441 Free PMC article.
-
Cocaine-induced plasticity in the cerebellum of sensitised mice.Psychopharmacology (Berl). 2015 Dec;232(24):4455-67. doi: 10.1007/s00213-015-4072-1. Epub 2015 Oct 20. Psychopharmacology (Berl). 2015. PMID: 26482898
-
Involvement of tissue plasminogen activator in stress responsivity during acute cocaine withdrawal in mice.Stress. 2010 Nov;13(6):481-90. doi: 10.3109/10253891003786415. Epub 2010 Jul 28. Stress. 2010. PMID: 20666641 Free PMC article.
-
Combination of Clinically Utilized Kappa-Opioid Receptor Agonist Nalfurafine With Low-Dose Naltrexone Reduces Excessive Alcohol Drinking in Male and Female Mice.Alcohol Clin Exp Res. 2019 Jun;43(6):1077-1090. doi: 10.1111/acer.14033. Epub 2019 May 2. Alcohol Clin Exp Res. 2019. PMID: 30908671 Free PMC article.
-
Methamphetamine-associated cleavage of the synaptic adhesion molecule intercellular adhesion molecule-5.J Neurochem. 2011 Aug;118(4):521-32. doi: 10.1111/j.1471-4159.2010.07153.x. Epub 2011 Jan 19. J Neurochem. 2011. PMID: 21166806 Free PMC article.
References
-
- Nestler EJ. Historical review: molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol Sci. 2004;25(4):210–218. - PubMed
-
- Kreek MJ, LaForge KS, Butelman E. Pharmacotherapy of addictions. Nat Rev Drug Discov. 2002;1(9):710–726. - PubMed
-
- Spangler R, Unterwald EM, Kreek MJ. ‘Binge’ cocaine administration induces a sustained increase of prodynorphin mRNA in rat caudate-putamen. Brain Res Mol Brain Res. 1993;19(4):323–327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

