Identification and specific localization of tyrosine-phosphorylated proteins in Trypanosoma brucei
- PMID: 19181871
- PMCID: PMC2669198
- DOI: 10.1128/EC.00366-08
Identification and specific localization of tyrosine-phosphorylated proteins in Trypanosoma brucei
Abstract
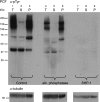
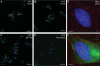
Phosphorylation on tyrosine residues is a key signal transduction mechanism known to regulate intercellular and intracellular communication in multicellular organisms. Despite the lack of conventional tyrosine kinases in the genome of the single cell organism Trypanosoma brucei, phosphorylation on trypanosomal protein tyrosine residues has been reported for this parasite. However, the identities of most of the tyrosine-phosphorylated proteins and their precise site(s) of phosphorylation were unknown. Here, we have applied a phosphotyrosine-specific proteomics approach to identify 34 phosphotyrosine-containing proteins from whole-cell extracts of procyclic form T. brucei. A significant proportion of the phosphotyrosine-containing proteins identified in this study were protein kinases of the CMGC kinase group as well as some proteins of unknown function and proteins involved in energy metabolism, protein synthesis, and RNA metabolism. Interestingly, immunofluorescence microscopy using anti-phosphotyrosine antibodies suggests that there is a concentration of tyrosine-phosphorylated proteins associated with cytoskeletal structures (basal body and flagellum) and in the nucleolus of the parasite. This localization of tyrosine-phosphorylated proteins supports the idea that the function of signaling molecules is controlled by their precise location in T. brucei, a principle well known from higher eukaryotes.
Figures





Similar articles
-
Global quantitative SILAC phosphoproteomics reveals differential phosphorylation is widespread between the procyclic and bloodstream form lifecycle stages of Trypanosoma brucei.J Proteome Res. 2013 May 3;12(5):2233-44. doi: 10.1021/pr400086y. Epub 2013 Mar 29. J Proteome Res. 2013. PMID: 23485197 Free PMC article.
-
Glycosome-associated tyrosine-phosphorylated protein in Trypanosoma brucei.Trop Med Parasitol. 1993 Dec;44(4):281-4. Trop Med Parasitol. 1993. PMID: 8134768
-
Developmental regulation of pp44/46, tyrosine-phosphorylated proteins associated with tyrosine/serine kinase activity in Trypanosoma brucei.Mol Biochem Parasitol. 1994 Jan;63(1):69-78. doi: 10.1016/0166-6851(94)90009-4. Mol Biochem Parasitol. 1994. PMID: 8183324
-
Distinct patterns of tyrosine phosphorylation during the life cycle of Trypanosoma brucei.Mol Biochem Parasitol. 1991 Apr;45(2):241-8. doi: 10.1016/0166-6851(91)90091-j. Mol Biochem Parasitol. 1991. PMID: 1710035
-
The phosphoproteome of bloodstream form Trypanosoma brucei, causative agent of African sleeping sickness.Mol Cell Proteomics. 2009 Jul;8(7):1527-38. doi: 10.1074/mcp.M800556-MCP200. Epub 2009 Apr 4. Mol Cell Proteomics. 2009. PMID: 19346560 Free PMC article.
Cited by
-
Rewiring and regulation of cross-compartmentalized metabolism in protists.Philos Trans R Soc Lond B Biol Sci. 2010 Mar 12;365(1541):831-45. doi: 10.1098/rstb.2009.0259. Philos Trans R Soc Lond B Biol Sci. 2010. PMID: 20124348 Free PMC article. Review.
-
The phosphoproteomes of Plasmodium falciparum and Toxoplasma gondii reveal unusual adaptations within and beyond the parasites' boundaries.Cell Host Microbe. 2011 Oct 20;10(4):410-9. doi: 10.1016/j.chom.2011.09.004. Cell Host Microbe. 2011. PMID: 22018241 Free PMC article.
-
Defeating the trypanosomatid trio: proteomics of the protozoan parasites causing neglected tropical diseases.RSC Med Chem. 2020 May 22;11(6):625-645. doi: 10.1039/d0md00122h. eCollection 2020 Jun 1. RSC Med Chem. 2020. PMID: 33479664 Free PMC article. Review.
-
Deep evolutionary conservation of an intramolecular protein kinase activation mechanism.PLoS One. 2012;7(1):e29702. doi: 10.1371/journal.pone.0029702. Epub 2012 Jan 3. PLoS One. 2012. PMID: 22235329 Free PMC article.
-
The Trypanosoma brucei life cycle switch TbPTP1 is structurally conserved and dephosphorylates the nucleolar protein NOPP44/46.J Biol Chem. 2010 Jul 16;285(29):22075-81. doi: 10.1074/jbc.M110.108860. Epub 2010 May 5. J Biol Chem. 2010. PMID: 20444707 Free PMC article.
References
-
- Bakalara, N., A. Seyfang, T. Baltz, and C. Davis. 1995. Trypanosoma brucei and Trypanosoma cruzi: life cycle-regulated protein tyrosine phosphatase activity. Exp. Parasitol. 81302-312. - PubMed
-
- Broadhead, R., H. R. Dawe, H. Farr, S. Griffiths, S. R. Hart, N. Portman, M. K. Shaw, M. L. Ginger, S. J. Gaskell, P. G. McKean, and K. Gull. 2006. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature 440224-227. - PubMed
-
- Brun, R., and Schönenberger. 1979. Cultivation and in vitro cloning or procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Acta Trop. 36289-292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

