Restoration of contractility in hyperhomocysteinemia by cardiac-specific deletion of NMDA-R1
- PMID: 19181966
- PMCID: PMC2660230
- DOI: 10.1152/ajpheart.00750.2008
Restoration of contractility in hyperhomocysteinemia by cardiac-specific deletion of NMDA-R1
Abstract
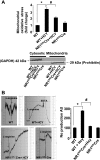
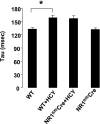
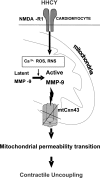
Homocysteine (HCY) activated mitochondrial matrix metalloproteinase-9 and led to cardiomyocyte dysfunction, in part, by inducing mitochondrial permeability (MPT). Treatment with MK-801 [N-methyl-d-aspartate (NMDA) receptor antagonist] ameliorated the HCY-induced decrease in myocyte contractility. However, the role of cardiomyocyte NMDA-receptor 1 (R1) activation in hyperhomocysteinemia (HHCY) leading to myocyte dysfunction was not well understood. We tested the hypothesis that the cardiac-specific deletion of NMDA-R1 mitigated the HCY-induced decrease in myocyte contraction, in part, by decreasing nitric oxide (NO). Cardiomyocyte-specific knockout of NMDA-R1 was generated using cre/lox technology. NMDA-R1 expression was detected by Western blot and confocal microscopy. MPT was determined using a spectrophotometer. Myocyte contractility and calcium transients were studied using the IonOptix video-edge detection system and fura 2-AM loading. We observed that HHCY induced NO production by agonizing NMDA-R1. HHCY induced the MPT by agonizing NMDA-R1. HHCY caused a decrease in myocyte contractile performance, maximal rate of contraction and relaxation, and prolonged the time to 90% peak shortening and 90% relaxation by agonizing NMDA-R1. HHCY decreased contraction amplitude with the increase in calcium concentration. The recovery of calcium transient was prolonged in HHCY mouse myocyte by agonizing NMDA-R1. It was suggested that HHCY increased mitochondrial NO levels and induced MPT, leading to the decline in myocyte mechanical function by agonizing NMDA-R1.
Figures




Similar articles
-
Mitochondrial mitophagic mechanisms of myocardial matrix metabolism and remodelling.Arch Physiol Biochem. 2012 Feb;118(1):31-42. doi: 10.3109/13813455.2011.635660. Epub 2011 Dec 19. Arch Physiol Biochem. 2012. PMID: 22181043 Free PMC article. Review.
-
Mitochondrial matrix metalloproteinase activation decreases myocyte contractility in hyperhomocysteinemia.Am J Physiol Heart Circ Physiol. 2008 Aug;295(2):H890-7. doi: 10.1152/ajpheart.00099.2008. Epub 2008 Jun 20. Am J Physiol Heart Circ Physiol. 2008. PMID: 18567713 Free PMC article.
-
Cardiac specific deletion of N-methyl-d-aspartate receptor 1 ameliorates mtMMP-9 mediated autophagy/mitophagy in hyperhomocysteinemia.J Recept Signal Transduct Res. 2010 Apr;30(2):78-87. doi: 10.3109/10799891003614808. J Recept Signal Transduct Res. 2010. PMID: 20170426 Free PMC article.
-
Mitochondrial MMP activation, dysfunction and arrhythmogenesis in hyperhomocysteinemia.Curr Vasc Pharmacol. 2008 Apr;6(2):84-92. doi: 10.2174/157016108783955301. Curr Vasc Pharmacol. 2008. PMID: 18393909 Review.
-
Homocysteine induces cardiomyocyte dysfunction and apoptosis through p38 MAPK-mediated increase in oxidant stress.J Mol Cell Cardiol. 2012 Mar;52(3):753-60. doi: 10.1016/j.yjmcc.2011.12.009. Epub 2011 Dec 29. J Mol Cell Cardiol. 2012. PMID: 22227328 Free PMC article.
Cited by
-
Direct Cardiac Actions of the Sodium Glucose Co-Transporter 2 Inhibitor Empagliflozin Improve Myocardial Oxidative Phosphorylation and Attenuate Pressure-Overload Heart Failure.J Am Heart Assoc. 2021 Mar 16;10(6):e018298. doi: 10.1161/JAHA.120.018298. Epub 2021 Mar 13. J Am Heart Assoc. 2021. PMID: 33719499 Free PMC article.
-
Restoration of Cardiac Function After Myocardial Infarction by Long-Term Activation of the CNS Leptin-Melanocortin System.JACC Basic Transl Sci. 2021 Jan 25;6(1):55-70. doi: 10.1016/j.jacbts.2020.11.007. eCollection 2021 Jan. JACC Basic Transl Sci. 2021. PMID: 33532666 Free PMC article.
-
Electrical stimulation of cardiomyocytes activates mitochondrial matrix metalloproteinase causing electrical remodeling.Biochem Biophys Res Commun. 2011 Jan 21;404(3):762-6. doi: 10.1016/j.bbrc.2010.12.039. Epub 2010 Dec 16. Biochem Biophys Res Commun. 2011. PMID: 21167815 Free PMC article.
-
Mitochondrial mitophagic mechanisms of myocardial matrix metabolism and remodelling.Arch Physiol Biochem. 2012 Feb;118(1):31-42. doi: 10.3109/13813455.2011.635660. Epub 2011 Dec 19. Arch Physiol Biochem. 2012. PMID: 22181043 Free PMC article. Review.
-
The effects of homocysteine-related compounds on cardiac contractility, coronary flow, and oxidative stress markers in isolated rat heart.Mol Cell Biochem. 2012 Nov;370(1-2):59-67. doi: 10.1007/s11010-012-1398-4. Epub 2012 Jul 22. Mol Cell Biochem. 2012. PMID: 22821198
References
-
- Albert CM, Rifai N, Stampfer MJ, Ridker PM. Prospective study of C-reactive protein, Hcy and plasma lipid levels as predictors of SCD. Circulation 105: 2595–2599, 2002. - PubMed
-
- Bollani G, Ferrari R, Bersatti F, Ferrari M, Cattaneo M, Zighetti MI, Visioli O, Sanelli D. A hyperhomocysteinemia study in a population with a familial factor for acute MI and SCD at a young age. Cardiologia 44: 75–81, 1999. - PubMed
-
- Burke AP, Fonseca V, Kolodgie F, Zieske A, Fink L, Virmani R. Increased serum Hcy and SCD resulting from coronary atherosclerosis with fibrous plaques. Arterioscler Thromb Vasc Biol 22: 1936–1941, 2002. - PubMed
-
- Cui G, Esmailian F, Plunkett M, Marelli D, Ardehali A, Odim J, Laks H, Sen L. Atrial extracellular matrix remodeling and the maintenance of atrial fibrillation. Circulation 109: 363–368, 2004. - PubMed
-
- Gao X, Xu XB, Pang J, Zhang C, Ding JM, Peng X, Liu Y, Cao JM. NMDA receptor activation induces mitochondrial dysfunction, oxidative stress and apoptosis in cultured neonatal rat cardiomyocytes. Physiol Res 56: 559–569, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

