Aging and diabetes impair the neovascular potential of adipose-derived stromal cells
- PMID: 19182604
- PMCID: PMC2878769
- DOI: 10.1097/PRS.0b013e3181954d08
Aging and diabetes impair the neovascular potential of adipose-derived stromal cells
Abstract
Background: Aging and diabetes are major risk factors for poor wound healing and tissue regeneration that reflect an impaired ability to respond to ischemic insults. The authors explored the intrinsic neovascular potential of adipose-derived stromal cells in the setting of advanced age and in type 1 and type 2 diabetes.
Methods: Adipose-derived stromal cells isolated from young, aged, streptozotocin-induced, and db/db diabetic mice were exposed to normoxia and hypoxia in vitro. Vascular endothelial growth factor (VEGF) expression, proliferation, and tubulization were measured. Conditioned media harvested from adipose-derived stromal cell cultures were assessed for their ability to stimulate human umbilical vein endothelial cell proliferation (n = 3 and n = 3).
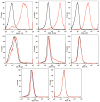
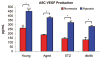
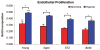
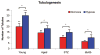
Results: Young adipose-derived stromal cells demonstrated significantly higher levels of VEGF production, proliferation, and tubulogenesis than those derived from aged, streptozotocin-induced, and db/db mice in both normoxia and hypoxia. Although aged and diabetic adipose-derived stromal cells retained the ability to up-regulate VEGF secretion, proliferation, and tubulogenesis in response to hypoxia, the response was blunted compared with young controls. Conditioned media derived from these cells cultured in normoxia in vitro also had a significantly greater ability to increase human umbilical vein endothelial cell proliferation compared with media harvested from aged, streptozotocin-induced, and db/db adipose-derived stromal cells. This effect was magnified in conditioned media harvested from hypoxic adipose-derived stromal cell cultures.
Conclusions: This study demonstrates that aging and type 1 and type 2 diabetes impair intrinsic adipose-derived stromal cell function; however, these cells may still be a suitable source of angiogenic cells that can potentially improve neovascularization of ischemic tissues.
Conflict of interest statement
Figures
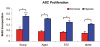

Similar articles
-
Neovascular potential of adipose-derived stromal cells (ASCs) from diabetic patients.Wound Repair Regen. 2012 Mar-Apr;20(2):243-52. doi: 10.1111/j.1524-475X.2012.00765.x. Epub 2012 Feb 14. Wound Repair Regen. 2012. PMID: 22332670
-
IFATS collection: Adipose stromal cells adopt a proangiogenic phenotype under the influence of hypoxia.Stem Cells. 2009 Jan;27(1):266-74. doi: 10.1634/stemcells.2008-0276. Stem Cells. 2009. PMID: 18974212
-
Angiogenic capacity of human adipose-derived stromal cells during adipogenic differentiation: an in vitro study.Tissue Eng Part A. 2009 Feb;15(2):445-52. doi: 10.1089/ten.tea.2007.0429. Tissue Eng Part A. 2009. PMID: 18652540
-
Adipose-derived mesenchymal stromal cells from aged patients with coronary artery disease keep mesenchymal stromal cell properties but exhibit characteristics of aging and have impaired angiogenic potential.Stem Cells Transl Med. 2014 Jan;3(1):32-41. doi: 10.5966/sctm.2013-0014. Epub 2013 Dec 18. Stem Cells Transl Med. 2014. PMID: 24353175 Free PMC article.
-
Myoblast-conditioned media improve regeneration and revascularization of ischemic muscles in diabetic mice.Stem Cell Res Ther. 2015 Apr 12;6(1):61. doi: 10.1186/s13287-015-0063-8. Stem Cell Res Ther. 2015. PMID: 25889676 Free PMC article.
Cited by
-
Acute liver failure: A systematic review and network meta-analysis of optimal type of stem cells in animal models.World J Stem Cells. 2023 Jan 26;15(1):1-15. doi: 10.4252/wjsc.v15.i1.1. World J Stem Cells. 2023. PMID: 36713788 Free PMC article.
-
Comparison of Markers and Functional Attributes of Human Adipose-Derived Stem Cells and Dedifferentiated Adipocyte Cells from Subcutaneous Fat of an Obese Diabetic Donor.Adv Wound Care (New Rochelle). 2014 Mar 1;3(3):219-228. doi: 10.1089/wound.2013.0452. Adv Wound Care (New Rochelle). 2014. PMID: 24669358 Free PMC article.
-
Deferoxamine preconditioning to restore impaired HIF-1α-mediated angiogenic mechanisms in adipose-derived stem cells from STZ-induced type 1 diabetic rats.Cell Prolif. 2015 Oct;48(5):532-49. doi: 10.1111/cpr.12209. Cell Prolif. 2015. PMID: 26332145 Free PMC article.
-
Full title: High glucose protects mesenchymal stem cells from metformin-induced apoptosis through the AMPK-mediated mTOR pathway.Sci Rep. 2019 Nov 28;9(1):17764. doi: 10.1038/s41598-019-54291-y. Sci Rep. 2019. PMID: 31780804 Free PMC article.
-
Nanofiber Microenvironment Effectively Restores Angiogenic Potential of Diabetic Endothelial Cells.Adv Wound Care (New Rochelle). 2014 Nov 1;3(11):717-728. doi: 10.1089/wound.2013.0511. Adv Wound Care (New Rochelle). 2014. PMID: 25371854 Free PMC article.
References
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. - PubMed
-
- Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1626. - PubMed
-
- Wagner W, Wein F, Seckinger A. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402. - PubMed
-
- Kern S, Eichler H, Stroeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

