Intra-retinal visual cycle required for rapid and complete cone dark adaptation
- PMID: 19182795
- PMCID: PMC2707787
- DOI: 10.1038/nn.2258
Intra-retinal visual cycle required for rapid and complete cone dark adaptation
Abstract
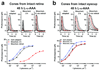
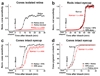
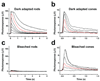
Daytime vision is mediated by retinal cones, which, unlike rods, remain functional even in bright light and dark-adapt rapidly. These cone properties are enabled by rapid regeneration of their pigment. This in turn requires rapid chromophore recycling that may not be achieved by the canonical retinal pigment epithelium visual cycle. Recent biochemical studies have suggested the presence of a second, cone-specific visual cycle, although its physiological function remains to be established. We found that the Müller cells in the salamander neural retina promote cone-specific pigment regeneration and dark adaptation that are independent of the pigment epithelium. Without this pathway, dark adaptation of cones was slow and incomplete. Notably, the rates of cone pigment regeneration by the retina and pigment epithelium visual cycles were essentially identical, suggesting a possible common rate-limiting step. Finally, we also observed cone dark adaptation in the isolated mouse retina.
Figures

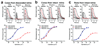

References
-
- Ebrey T, Koutalos Y. Vertebrate photoreceptors. Prog Retin Eye Res. 2001;20:49–94. - PubMed
-
- Palczewski K, et al. Rod outer segment retinol dehydrogenase: substrate specificity and role in phototransduction. Biochemistry. 1994;33:13741–13750. - PubMed
-
- Pepperberg DR, et al. Interphotoreceptor retinoid-binding protein (IRBP). Molecular biology and physiological role in the visual cycle of rhodopsin. Mol Neurobiol. 1993;7:61–85. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

