Helix movement is coupled to displacement of the second extracellular loop in rhodopsin activation
- PMID: 19182802
- PMCID: PMC2705779
- DOI: 10.1038/nsmb.1549
Helix movement is coupled to displacement of the second extracellular loop in rhodopsin activation
Abstract
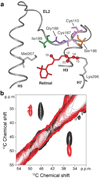
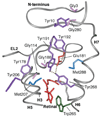
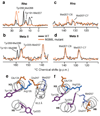
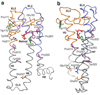
The second extracellular loop (EL2) of rhodopsin forms a cap over the binding site of its photoreactive 11-cis retinylidene chromophore. A crucial question has been whether EL2 forms a reversible gate that opens upon activation or acts as a rigid barrier. Distance measurements using solid-state (13)C NMR spectroscopy between the retinal chromophore and the beta4 strand of EL2 show that the loop is displaced from the retinal binding site upon activation, and there is a rearrangement in the hydrogen-bonding networks connecting EL2 with the extracellular ends of transmembrane helices H4, H5 and H6. NMR measurements further reveal that structural changes in EL2 are coupled to the motion of helix H5 and breaking of the ionic lock that regulates activation. These results provide a comprehensive view of how retinal isomerization triggers helix motion and activation in this prototypical G protein-coupled receptor.
Figures


References
-
- Samson M, et al. The second extracellular loop of CCR5 is the major determinant of ligand specificity. J. Biol. Chem. 1997;272:24934–24941. - PubMed
-
- Klco JM, Wiegand CB, Narzinski K, Baranski TJ. Essential role for the second extracellular loop in C5a receptor activation. Nat. Struct. Mol. Biol. 2005;12:320–326. - PubMed
-
- Scarselli M, Li B, Kim SK, Wess J. Multiple residues in the second extracellular loop are critical for M-3 muscarinic acetylcholine receptor activation. J. Biol. Chem. 2007;282:7385–7396. - PubMed
-
- Palczewski K, et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

