The peptidyl-prolyl isomerase Pin1 facilitates cytokine-induced survival of eosinophils by suppressing Bax activation
- PMID: 19182807
- PMCID: PMC2847832
- DOI: 10.1038/ni.1697
The peptidyl-prolyl isomerase Pin1 facilitates cytokine-induced survival of eosinophils by suppressing Bax activation
Abstract
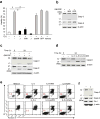
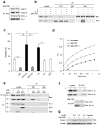
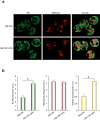
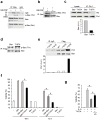
The mechanisms by which cytokine signals prevent the activation and mitochondrial targeting of the proapoptotic protein Bax are unclear. Here we show, using primary human eosinophils, that in the absence of the prosurvival cytokines granulocyte-macrophage colony-stimulating factor and interleukin 5, Bax spontaneously underwent activation and initiated mitochondrial disruption. Inhibition of Bax resulted in less eosinophil apoptosis, even in the absence of cytokines. Granulocyte-macrophage colony-stimulating factor induced activation of the kinase Erk1/2, which phosphorylated Thr167 of Bax; this facilitated new interaction of Bax with the prolyl isomerase Pin1. Blockade of Pin1 led to cleavage and mitochondrial translocation of Bax and caspase activation, regardless of the presence of cytokines. Our findings indicate that Pin1 is a key mediator of prosurvival signaling and is a regulator of Bax function.
Conflict of interest statement
Figures

Similar articles
-
Determinants of eosinophil survival and apoptotic cell death.Apoptosis. 2015 Feb;20(2):224-34. doi: 10.1007/s10495-014-1072-2. Apoptosis. 2015. PMID: 25563855 Free PMC article. Review.
-
Pin1-FADD interactions regulate Fas-mediated apoptosis in activated eosinophils.J Immunol. 2013 May 15;190(10):4937-45. doi: 10.4049/jimmunol.1202646. Epub 2013 Apr 19. J Immunol. 2013. PMID: 23606538 Free PMC article.
-
The peptidyl-prolyl isomerase Pin1 regulates the stability of granulocyte-macrophage colony-stimulating factor mRNA in activated eosinophils.Nat Immunol. 2005 Dec;6(12):1280-7. doi: 10.1038/ni1266. Epub 2005 Nov 6. Nat Immunol. 2005. PMID: 16273101
-
Pin1 promotes cell death in NGF-dependent neurons through a mechanism requiring c-Jun activity.J Neurochem. 2008 Jul;106(2):734-45. doi: 10.1111/j.1471-4159.2008.05427.x. Epub 2008 Apr 14. J Neurochem. 2008. PMID: 18419764 Free PMC article.
-
Eosinophil priming by cytokines: from cellular signal to in vivo modulation.Eur Respir J Suppl. 1996 Aug;22:119s-125s. Eur Respir J Suppl. 1996. PMID: 8871056 Review.
Cited by
-
Determinants of eosinophil survival and apoptotic cell death.Apoptosis. 2015 Feb;20(2):224-34. doi: 10.1007/s10495-014-1072-2. Apoptosis. 2015. PMID: 25563855 Free PMC article. Review.
-
Inhibition of the prolyl isomerase Pin1 enhances the ability of sorafenib to induce cell death and inhibit tumor growth in hepatocellular carcinoma.Oncotarget. 2017 May 2;8(18):29771-29784. doi: 10.18632/oncotarget.15967. Oncotarget. 2017. PMID: 28404959 Free PMC article.
-
The prolyl isomerase Pin1 acts as a novel molecular switch for TNF-alpha-induced priming of the NADPH oxidase in human neutrophils.Blood. 2010 Dec 23;116(26):5795-802. doi: 10.1182/blood-2010-03-273094. Epub 2010 Oct 18. Blood. 2010. PMID: 20956805 Free PMC article.
-
Sensitization of melanoma cells for TRAIL-induced apoptosis by BMS-345541 correlates with altered phosphorylation and activation of Bax.Cell Death Dis. 2013 Jan 24;4(1):e477. doi: 10.1038/cddis.2012.198. Cell Death Dis. 2013. PMID: 23348591 Free PMC article.
-
Targeting Pin1 for Modulation of Cell Motility and Cancer Therapy.Biomedicines. 2021 Mar 31;9(4):359. doi: 10.3390/biomedicines9040359. Biomedicines. 2021. PMID: 33807199 Free PMC article. Review.
References
-
- Gibson PG, Saltos N, Borgas T. Airway mast cells and eosinophils correlate with clinical severity and airway hyperresponsiveness in corticosteroid-treated asthma. J Allergy Clin Immunol. 2000;105:752–759. - PubMed
-
- Humbles AA, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

