Mycoplasma pneumoniae J-domain protein required for terminal organelle function
- PMID: 19183275
- PMCID: PMC5833977
- DOI: 10.1111/j.1365-2958.2009.06602.x
Mycoplasma pneumoniae J-domain protein required for terminal organelle function
Abstract
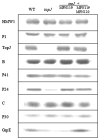
The cell wall-less prokaryote Mycoplasma pneumoniae causes tracheobronchitis and primary atypical pneumonia in humans. Colonization of the respiratory epithelium requires proper assembly of a complex, multifunctional, polar terminal organelle. Loss of a predicted J-domain protein also having domains unique to mycoplasma terminal organelle proteins (TopJ) resulted in a non-motile, adherence-deficient phenotype. J-domain proteins typically stimulate ATPase activity of Hsp70 chaperones to bind nascent peptides for proper folding, translocation or macromolecular assembly, or to resolve stress-induced protein aggregates. By Western immunoblotting all defined terminal organelle proteins examined except protein P24 remained at wild-type levels in the topJ mutant; previous studies established that P24 is required for normal initiation of terminal organelle formation. Nevertheless, terminal organelle proteins P1, P30, HMW1 and P41 failed to localize to a cell pole, and when evaluated quantitatively, P30 and HMW1 foci were undetectable in >40% of cells. Complementation of the topJ mutant with the recombinant wild-type topJ allele largely restored terminal organelle development, gliding motility and cytadherence. We propose that this J-domain protein, which localizes to the base of the terminal organelle in wild-type M. pneumoniae, functions in the late stages of assembly, positioning, or both, of nascent terminal organelles.
Figures





Similar articles
-
Proteins P24 and P41 function in the regulation of terminal-organelle development and gliding motility in Mycoplasma pneumoniae.J Bacteriol. 2007 Oct;189(20):7442-9. doi: 10.1128/JB.00867-07. Epub 2007 Aug 10. J Bacteriol. 2007. PMID: 17693502 Free PMC article.
-
Functional domain analysis of the Mycoplasma pneumoniae co-chaperone TopJ.Mol Microbiol. 2010 Jul 1;77(1):158-69. doi: 10.1111/j.1365-2958.2010.07196.x. Epub 2010 May 12. Mol Microbiol. 2010. PMID: 20487283 Free PMC article.
-
Loss of co-chaperone TopJ impacts adhesin P1 presentation and terminal organelle maturation in Mycoplasma pneumoniae.Mol Microbiol. 2011 Jul;81(2):528-39. doi: 10.1111/j.1365-2958.2011.07712.x. Epub 2011 Jun 23. Mol Microbiol. 2011. PMID: 21631602 Free PMC article.
-
Structure, function, and assembly of the terminal organelle of Mycoplasma pneumoniae.FEMS Microbiol Lett. 2001 Apr 20;198(1):1-7. doi: 10.1111/j.1574-6968.2001.tb10610.x. FEMS Microbiol Lett. 2001. PMID: 11325545 Review.
-
Mycoplasma pneumoniae cytadherence: organization and assembly of the attachment organelle.Trends Microbiol. 1998 Jan;6(1):15-8. doi: 10.1016/S0966-842X(97)01168-2. Trends Microbiol. 1998. PMID: 9481818 Review.
Cited by
-
Biological database of images and genomes: tools for community annotations linking image and genomic information.Database (Oxford). 2013 Apr 2;2013:bat016. doi: 10.1093/database/bat016. Print 2013. Database (Oxford). 2013. PMID: 23550062 Free PMC article.
-
Identification of Mycoplasma pneumoniae proteins interacting with NOD2 and their role in macrophage inflammatory response.Front Microbiol. 2024 May 28;15:1391453. doi: 10.3389/fmicb.2024.1391453. eCollection 2024. Front Microbiol. 2024. PMID: 38863748 Free PMC article.
-
Quantitative essentiality in a reduced genome: a functional, regulatory and structural fitness map.Mol Syst Biol. 2025 Aug 13. doi: 10.1038/s44320-025-00133-1. Online ahead of print. Mol Syst Biol. 2025. PMID: 40804181
-
Systematic Structural Analyses of Attachment Organelle in Mycoplasma pneumoniae.PLoS Pathog. 2015 Dec 3;11(12):e1005299. doi: 10.1371/journal.ppat.1005299. eCollection 2015 Dec. PLoS Pathog. 2015. PMID: 26633540 Free PMC article.
-
Mycoplasma synoviae LP78 is a fibronectin/plasminogen binding protein, putative adhesion, and potential diagnostic antigen.Front Microbiol. 2024 Jan 9;14:1335658. doi: 10.3389/fmicb.2023.1335658. eCollection 2023. Front Microbiol. 2024. PMID: 38264482 Free PMC article.
References
-
- Balish MF, Krause DC. Cytadherence and the cytoskeleton. In: Herrmann SRaR., editor. Molecular Biology and Pathogenicity of the Mycoplasmas. New York: Kluwer Academic/Plenum Publishers; 2002. pp. 491–518.
-
- Balish MF, Santurri RT, Ricci AM, Lee KK, Krause DC. Localization of Mycoplasma pneumoniae cytadherence-associated protein HMW2 by fusion with green fluorescent protein: implications for attachment organelle structure. Mol Microbiol. 2003;47:49–60. - PubMed
-
- Baseman JB, Morrison-Plummer J, Drouillard D, Puleo-Scheppke B, Tryon VV, Holt SC. Identification of a 32-kilodalton protein of Mycoplasma pneumoniae associated with hemadsorption. Isr J Med Sci. 1987;23:474–479. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

