Form and function of damselfish skulls: rapid and repeated evolution into a limited number of trophic niches
- PMID: 19183467
- PMCID: PMC2654721
- DOI: 10.1186/1471-2148-9-24
Form and function of damselfish skulls: rapid and repeated evolution into a limited number of trophic niches
Abstract
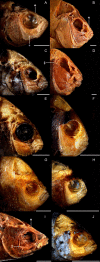
Background: Damselfishes (Perciformes, Pomacentridae) are a major component of coral reef communities, and the functional diversity of their trophic anatomy is an important constituent of the ecological morphology of these systems. Using shape analyses, biomechanical modelling, and phylogenetically based comparative methods, we examined the anatomy of damselfish feeding among all genera and trophic groups. Coordinate based shape analyses of anatomical landmarks were used to describe patterns of morphological diversity and determine positions of functional groups in a skull morphospace. These landmarks define the lever and linkage structures of the damselfish feeding system, and biomechanical analyses of this data were performed using the software program JawsModel4 in order to calculate the simple mechanical advantage (MA) employed by different skull elements during feeding, and to compute kinematic transmission coefficients (KT) that describe the efficiency with which angular motion is transferred through the complex linkages of damselfish skulls.
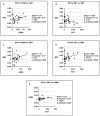
Results: Our results indicate that pomacentrid planktivores are significantly different from other damselfishes, that biting MA values and protrusion KT ratios are correlated with pomacentrid trophic groups more tightly than KT scores associated with maxillary rotation and gape angle, and that the MAs employed by their three biting muscles have evolved independently. Most of the biomechanical parameters examined have experienced low levels of phylogenetic constraint, which suggests that they have evolved quickly.
Conclusion: Joint morphological and biomechanical analyses of the same anatomical data provided two reciprocally illuminating arrays of information. Both analyses showed that the evolution of planktivory has involved important changes in pomacentrid functional morphology, and that the mechanics of upper jaw kinesis have been of great importance to the evolution of damselfish feeding. Our data support a tight and biomechanically defined link between structure and the functional ecology of fish skulls, and indicate that certain mechanisms for transmitting motion through their jaw linkages may require particular anatomical configurations, a conclusion that contravenes the concept of "many-to-one mapping" for fish jaw mechanics. Damselfish trophic evolution is characterized by rapid and repeated shifts between a small number of eco-morphological states, an evolutionary pattern that we describe as reticulate adaptive radiation.
Figures



Similar articles
-
The evolution of jaw protrusion mechanics is tightly coupled to bentho-pelagic divergence in damselfishes (Pomacentridae).J Exp Biol. 2017 Feb 15;220(Pt 4):652-666. doi: 10.1242/jeb.143115. Epub 2016 Dec 2. J Exp Biol. 2017. PMID: 27913600
-
Local phylogenetic divergence and global evolutionary convergence of skull function in reef fishes of the family Labridae.Proc Biol Sci. 2005 May 22;272(1567):993-1000. doi: 10.1098/rspb.2004.3013. Proc Biol Sci. 2005. PMID: 16024356 Free PMC article.
-
Phylogeny of the damselfishes (Pomacentridae) and patterns of asymmetrical diversification in body size and feeding ecology.PLoS One. 2021 Oct 27;16(10):e0258889. doi: 10.1371/journal.pone.0258889. eCollection 2021. PLoS One. 2021. PMID: 34705840 Free PMC article.
-
The role of cranial kinesis in birds.Comp Biochem Physiol A Mol Integr Physiol. 2001 Dec;131(1):197-205. doi: 10.1016/s1095-6433(01)00470-6. Comp Biochem Physiol A Mol Integr Physiol. 2001. PMID: 11733177 Review.
-
Craniofacial biomechanics: an overview of recent multibody modelling studies.J Anat. 2011 Jan;218(1):16-25. doi: 10.1111/j.1469-7580.2010.01317.x. Epub 2010 Nov 10. J Anat. 2011. PMID: 21062283 Free PMC article. Review.
Cited by
-
Diversity in rest-activity patterns among Lake Malawi cichlid fishes suggests a novel axis of habitat partitioning.J Exp Biol. 2021 Apr 1;224(7):jeb242186. doi: 10.1242/jeb.242186. Epub 2021 Apr 15. J Exp Biol. 2021. PMID: 33658242 Free PMC article.
-
A classic key innovation constrains oral jaw functional diversification in fishes.Evol Lett. 2024 Oct 8;9(1):24-40. doi: 10.1093/evlett/qrae046. eCollection 2025 Feb. Evol Lett. 2024. PMID: 39906576 Free PMC article.
-
Trait decoupling promotes evolutionary diversification of the trophic and acoustic system of damselfishes.Proc Biol Sci. 2014 Aug 22;281(1789):20141047. doi: 10.1098/rspb.2014.1047. Proc Biol Sci. 2014. PMID: 24990683 Free PMC article.
-
More than meets the eye: functionally salient changes in internal bone architecture accompany divergence in cichlid feeding mode.Int J Evol Biol. 2012;2012:538146. doi: 10.1155/2012/538146. Epub 2012 May 15. Int J Evol Biol. 2012. PMID: 22666625 Free PMC article.
-
The mitochondrial phylogeny of an ancient lineage of ray-finned fishes (Polypteridae) with implications for the evolution of body elongation, pelvic fin loss, and craniofacial morphology in Osteichthyes.BMC Evol Biol. 2010 Jan 25;10:21. doi: 10.1186/1471-2148-10-21. BMC Evol Biol. 2010. PMID: 20100320 Free PMC article.
References
-
- Wainwright PC. Functional Morphology as a Tool in Ecological Research. In: Wainwright PC, Reilly SM, editor. Ecological Morphology. Chicago: The University of Chicago Press; 1994. pp. 42–59.
-
- Westneat MW. Transmission of Force and Velocity in the Feeding Mechanisms of Labrid Fishes (Teleostei, Perciformes) Zoomorphology. 1994;114:103–118. doi: 10.1007/BF00396643. - DOI
-
- Westneat MW, Alfaro ME, Wainwright PC, Bellwood DR, Grubich JR, Fessler JL, Clements KD, Smith LL. Local phylogenetic divergence and global evolutionary convergence of skull function in reef fishes of the family Labridae. Proceedings of the Royal Society B-Biological Sciences. 2005;272:993–1000. doi: 10.1098/rspb.2004.3013. - DOI - PMC - PubMed
-
- Wainwright PC, Bellwood DR, Westneat MW, Grubich JR, Hoey AS. A functional morphospace for the skull of labrid fishes: patterns of diversity in a complex biomechanical system. Biological Journal of the Linnean Society. 2004;82:1–25. doi: 10.1111/j.1095-8312.2004.00313.x. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

