Nitric oxide administration using an oxygen hood: a pilot trial
- PMID: 19183804
- PMCID: PMC2629563
- DOI: 10.1371/journal.pone.0004312
Nitric oxide administration using an oxygen hood: a pilot trial
Abstract
Background: We have shown earlier that inhaled nitric oxide (iNO) administered by oxygen hood reduces pulmonary hypertension in an animal model (J Perinatol 2002; 22:50-6). Our objective in this study was to determine feasibility of iNO by oxygen hood in neonates with elevated alveolar-arterial oxygen gradients (A-aDO(2)).
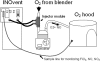
Methods/principal findings: Masked randomized controlled pilot trial. Inclusion criteria were: gestation>or=34 weeks, age<7 days, with post-ductal arterial line, and A-aDO(2) 400-600. Infants were randomized to study gas (iNO 20 ppm or equivalent O(2) flow) for 1 hr which was then weaned over the next 4 hours. Primary outcome was PaO(2) one hour post-randomization. Four infants each were randomized to iNO or O(2) (controls). Two of the four infants given iNO had an increase in PaO(2) of >100 torr, while oxygenation was unchanged in the controls. Methemoglobinemia and other adverse effects were not noted in any infant. Environmental levels of NO and NO(2) were minimal (<1 ppm) at >0.3 m from the hood.
Conclusions: Administration of iNO by oxygen hood is feasible. Larger randomized controlled trials are required to measure the efficacy and determine an appropriate target population for this technique.
Trial registration: ClinicalTrials.gov NCT00041548.
Conflict of interest statement
Figures

References
-
- Walsh-Sukys MC, Tyson JE, Wright LL, Bauer CR, Korones SB, et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics. 2000;105:14–20. - PubMed
-
- Finer NN, Barrington KJ. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst Rev 2001 (4). CD000399. 2001. DOI: 10.1002/14651858.CD000399. Accessed July 9, 2008. - PubMed
-
- Wessel DL, Adatia I, Van Marter LJ, Thompson JE, Kane JW, et al. Improved oxygenation in a randomized trial of inhaled nitric oxide for persistent pulmonary hypertension of the newborn. Pediatrics 100:e7. 1997. Available online at http://www.pediatrics.org/cgi/content/full/100/5/e7. Accessed July 9, 2008. - PubMed
-
- Roberts JD, Fineman JR, Morin FC, 3rd, Shaul PW, Rimar S, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The Inhaled Nitric Oxide Study Group. N Engl J Med. 1997;336:605–610. - PubMed
-
- The Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl J Med. 1997;336:597–604. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

