Cloning and immunoreactivity of the 5-HT 1Mac and 5-HT 2Mac receptors in the central nervous system of the freshwater prawn Macrobrachium rosenbergii
- PMID: 19184976
- PMCID: PMC2719784
- DOI: 10.1002/cne.21979
Cloning and immunoreactivity of the 5-HT 1Mac and 5-HT 2Mac receptors in the central nervous system of the freshwater prawn Macrobrachium rosenbergii
Abstract
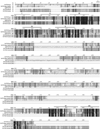
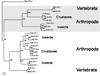
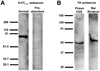
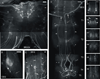
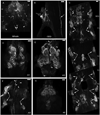
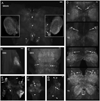
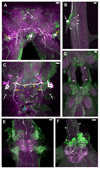
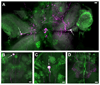
Biogenic amines are implicated in several mental disorders, many of which involve social interactions. Simple model systems, such as crustaceans, are often more amenable than vertebrates for studying mechanisms underlying behaviors. Although various cellular responses of biogenic amines have been characterized in crustaceans, the mechanisms linking these molecules to behavior remain largely unknown. Observed effects of serotonin receptor agonists and antagonists in abdomen posture, escape responses, and fighting have led to the suggestion that biogenic amine receptors may play a role in modulating interactive behaviors. As a first step in understanding this potential role of such receptors, we have cloned and fully sequenced two serotonin receptors, 5-HT(1Mac) and 5-HT(2Mac), from the CNS of the freshwater prawn Macrobrachium rosenbergii and have mapped their CNS immunohistochemical distribution. 5-HT(1Mac) was found primarily on the membranes of subsets of cells in all CNS ganglia, in fibers that traverse all CNS regions, and in the cytoplasm of a small number of cells in the brain and circum- and subesophageal ganglia (SEG), most of which also appear to contain dopamine. The pattern of 5-HT(2Mac) immunoreactivity was found to differ significantly; it was found mostly in the central neuropil area of all ganglia, in glomeruli of the brain's olfactory lobes, and in the cytoplasm of a small number of neurons in the SEG, thoracic, and some abdominal ganglia. The observed differences in terms of localization, distribution within cells, and intensity of immunoreactive staining throughout the prawn's CNS suggest that these receptors are likely to play different roles.
(c) 2009 Wiley-Liss, Inc.
Figures

References
-
- Adams CF, Liley NR, Gorzalka BB. PCPA increases aggression in male firemouth cichlids. Pharmacology. 1996;53(5):328–330. - PubMed
-
- Albert FW, Shchepina O, Winter C, Römpler H, Teupser D, Palme R, Ceglarek U, Kratzsch J, Sohr R, Trut LN, Thiery J, Morgenstern R, Plyusnina IZ, Schöneberg T, Pääbo S. Phenotypic differences in behavior, physiology and neurochemistry between rats selected for tameness and for defensive aggression towards humans. Horm Behav. 2008;53(3):413–421. - PubMed
-
- Barbas D, Zappulla JP, Angers S, Bouvier M. Functional characterization of a novel serotonin receptor (5-HTap2) expressed in the CNS of Aplysia californica. J Neurochem. 2002;80:335–345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS39103/NS/NINDS NIH HHS/United States
- R24 MH048190/MH/NIMH NIH HHS/United States
- R25 GM061838/GM/NIGMS NIH HHS/United States
- S06GM008224/GM/NIGMS NIH HHS/United States
- R21 RR025699/RR/NCRR NIH HHS/United States
- MH48190/MH/NIMH NIH HHS/United States
- G12 RR003051/RR/NCRR NIH HHS/United States
- S06 GM008224/GM/NIGMS NIH HHS/United States
- G12RR03051/RR/NCRR NIH HHS/United States
- R01 NS039103/NS/NINDS NIH HHS/United States
- G12 MD007600/MD/NIMHD NIH HHS/United States
- SC3 GM084763/GM/NIGMS NIH HHS/United States
- G12 RR003050/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

