Apotopes and the biliary specificity of primary biliary cirrhosis
- PMID: 19185000
- PMCID: PMC2665925
- DOI: 10.1002/hep.22736
Apotopes and the biliary specificity of primary biliary cirrhosis
Abstract
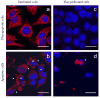
Primary biliary cirrhosis (PBC) is characterized by antimitochondrial antibodies (AMAs), directed to the E2 component of the pyruvate dehydrogenase complex (PDC-E2). Notwithstanding the presence of mitochondria in virtually all nucleated cells, the destruction in PBC is limited to small intrahepatic bile ducts. The reasons for this tissue specificity remain unknown, although biliary epithelial cells (BECs) uniquely preserve the PDC-E2 epitope following apoptosis. Notably, PBC recurs in an allogeneic transplanted liver, suggesting generic rather than host PBC-specific susceptibility of BEC. We used cultured human intrahepatic BECs (HIBECs) and other well-characterized cell lines, including, HeLa, CaCo-2 cells, and nontransformed human keratinocytes and bronchial epithelial cells, to determine the integrity and specific localization of PDC-E2 during induced apoptosis. All cell lines, both before and after apoptosis, were tested with sera from patients with PBC (n = 30), other autoimmune liver and rheumatic diseases (n = 20), and healthy individuals (n = 20) as well as with a mouse monoclonal antibody against PDC-E2 and AMA with an immunoglobulin A isotype. PDC-E2 was found to localize unmodified within apoptotic blebs of HIBECs, but not within blebs of various other cell lineages studied. The fact that AMA-containing sera reacted with PDC-E2 on apoptotic BECs without a requirement for permeabilization suggests that the autoantigen is accessible to the immune system during apoptosis.
Conclusion: Our data indicate that the tissue (cholangiocyte) specificity of the autoimmune injury in PBC is a consequence of the unique characteristics of HIBECs during apoptosis and can be explained by exposure to the immune system of intact immunoreactive PDC-E2 within apoptotic blebs.
Figures



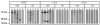
References
-
- Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. - PubMed
-
- Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7:964–974. - PubMed
-
- Torok NJ. Apoptotic cell death takes its toll. Hepatology. 2007;46:1323–1325. - PubMed
-
- Perniok A, Wedekind F, Herrmann M, Specker C, Schneider M. High levels of circulating early apoptic peripheral blood mononuclear cells in systemic lupus erythematosus. Lupus. 1998;7:113–118. - PubMed
-
- Ruiz-Arguelles A, Brito GJ, Reyes-Izquierdo P, Perez-Romano B, Sanchez-Sosa S. Apoptosis of melanocytes in vitiligo results from antibody penetration. J Autoimmun. 2007;29:281–286. - PubMed
