SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-kappaB pathway in human monocyte macrophages in vitro
- PMID: 19185596
- PMCID: PMC2699111
- DOI: 10.1016/j.virusres.2009.01.005
SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-kappaB pathway in human monocyte macrophages in vitro
Abstract
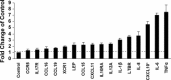
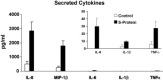
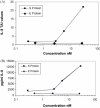
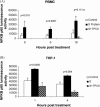
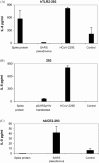
A purified recombinant spike (S) protein was studied for its effect on stimulating human peripheral blood monocyte macrophages (PBMC). We examined inflammatory gene mRNA abundances found in S protein-treated PBMC using gene arrays. We identified differential mRNA abundances of genes with functional properties associated with antiviral (CXCL10) and inflammatory (IL-6 and IL-8) responses. We confirmed cytokine mRNA increases by real-time quantitative(q) RT-PCR or ELISA. We further analyzed the sensitivity and specificity of the prominent IL-8 response. By real-time qRT-PCR, S protein was shown to stimulate IL-8 mRNA accumulation in a dose dependent manner while treatment with E protein did not. Also, titration of S protein-specific production and secretion of IL-8 by ELISA showed that the dose of 5.6nM of S produced a significant increase in IL-8 (p=0.003) compared to mock-treated controls. The increase in IL-8 stimulated by a concentration of 5.6nM of S was comparable to concentrations seen for S protein binding to ACE2 or to neutralizing monoclonal antibody suggesting a physiological relevance. An NF-kappaB inhibitor, TPCK (N-Tosyl-L-Phenylalanine Chloromethyl Ketone) could suppress IL-8 production and secretion in response to S protein in PBMC and THP-1 cells and in HCoV-229E virus-infected PBMC. Activation and translocation of NF-kappaB was shown to occur rapidly following exposure of PBMC or THP-1 cells to S protein using a highly sensitive assay for active nuclear NF-kappaB p65 transcription factor. The results further suggested that released or secreted S protein could activate blood monocytes through recognition by toll-like receptor (TLR)2 ligand.
Figures

Similar articles
-
Up-regulation of IL-6 and TNF-alpha induced by SARS-coronavirus spike protein in murine macrophages via NF-kappaB pathway.Virus Res. 2007 Sep;128(1-2):1-8. doi: 10.1016/j.virusres.2007.02.007. Epub 2007 May 25. Virus Res. 2007. PMID: 17532082 Free PMC article.
-
SARS patients-derived human recombinant antibodies to S and M proteins efficiently neutralize SARS-coronavirus infectivity.Biomed Environ Sci. 2005 Dec;18(6):363-74. Biomed Environ Sci. 2005. PMID: 16544518
-
T-cell epitopes in severe acute respiratory syndrome (SARS) coronavirus spike protein elicit a specific T-cell immune response in patients who recover from SARS.J Virol. 2004 Jun;78(11):5612-8. doi: 10.1128/JVI.78.11.5612-5618.2004. J Virol. 2004. PMID: 15140958 Free PMC article.
-
Human monoclonal antibody combination against SARS coronavirus: synergy and coverage of escape mutants.PLoS Med. 2006 Jul;3(7):e237. doi: 10.1371/journal.pmed.0030237. PLoS Med. 2006. PMID: 16796401 Free PMC article.
-
Induction of IL-8 release in lung cells via activator protein-1 by recombinant baculovirus displaying severe acute respiratory syndrome-coronavirus spike proteins: identification of two functional regions.J Immunol. 2004 Dec 15;173(12):7602-14. doi: 10.4049/jimmunol.173.12.7602. J Immunol. 2004. PMID: 15585888
Cited by
-
The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier.Neurobiol Dis. 2020 Dec;146:105131. doi: 10.1016/j.nbd.2020.105131. Epub 2020 Oct 11. Neurobiol Dis. 2020. PMID: 33053430 Free PMC article.
-
Herbo-mineral formulation, Divya-Swasari-Vati averts SARS-CoV-2 pseudovirus entry into human alveolar epithelial cells by interfering with spike protein-ACE 2 interaction and IL-6/TNF-α /NF-κB signaling.Front Pharmacol. 2022 Oct 26;13:1024830. doi: 10.3389/fphar.2022.1024830. eCollection 2022. Front Pharmacol. 2022. PMID: 36386162 Free PMC article.
-
Exploring the therapeutic potential of forkhead box O for outfoxing COVID-19.Open Biol. 2021 Jun;11(6):210069. doi: 10.1098/rsob.210069. Epub 2021 Jun 9. Open Biol. 2021. PMID: 34102081 Free PMC article. Review.
-
Extraordinary GU-rich single-strand RNA identified from SARS coronavirus contributes an excessive innate immune response.Microbes Infect. 2013 Feb;15(2):88-95. doi: 10.1016/j.micinf.2012.10.008. Epub 2012 Oct 30. Microbes Infect. 2013. PMID: 23123977 Free PMC article.
-
Lactoferrin Binding to SARS-CoV-2 Spike Glycoprotein Blocks Pseudoviral Entry and Relieves Iron Protein Dysregulation in Several In Vitro Models.Pharmaceutics. 2022 Oct 3;14(10):2111. doi: 10.3390/pharmaceutics14102111. Pharmaceutics. 2022. PMID: 36297546 Free PMC article.
References
-
- Barton G.M., Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;6:712–721. - PubMed
-
- Cameron M.J., Ran L., Xu L., Danesh A., Bermejo-Martin J.F., Cameron C.M., Muller M.P., Gold W.L., Richardson S.E., Poutanen S.M., Willey B.M., DeVries M.E., Fang Y., Seneviratne C., Bosinger S.E., Persad D., Wilkinson P., Greller L.D., Somogyi R., Humar A., Keshavjee S., Louie M., Loeb M.B., Brunton J., McGeer A.J., Kelvin D.J., The Canadian SARS Research Network Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J. Virol. 2007;81:8692–8706. - PMC - PubMed
-
- Chang Y.J., Liu C.Y.-Y., Chiang B.L., Chao Y.C., Chen C.C. Induction of IL-8 release in lung cells via activator protein-1 by recombinant baculovirus displaying severe acute respiratory syndrome-coronavirus spike proteins: identification of two functional regions. J. Immunol. 2004;173:7602–7614. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

