Dynamics of thin-filament activation in rabbit skeletal muscle fibers examined by time-resolved x-ray diffraction
- PMID: 19186142
- PMCID: PMC2716644
- DOI: 10.1016/j.bpj.2008.09.022
Dynamics of thin-filament activation in rabbit skeletal muscle fibers examined by time-resolved x-ray diffraction
Abstract
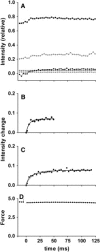
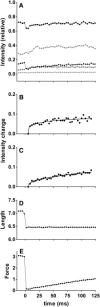
By using skinned-rabbit skeletal muscle fibers, the time courses of changes of thin filament-based x-ray reflections were followed at a 3.4-ms time resolution during thin-filament activation. To discriminate between the effects of calcium binding and myosin binding on thin-filament activity, measurements were performed after caged-calcium photolysis in fibers with full-filament or no-filament overlap, or during force recovery after a quick release. All three reflections examined, i.e., the second actin layer line (second ALL, reporting the tropomyosin movement), the sixth ALL (reporting actin structural change), and the meridional troponin reflections, exhibited calcium-induced and myosin-induced components, but their rate constants and polarities were different. Generally, calcium-induced components exhibited fast rate constants (>100 s(-1)). The myosin-induced components of the second ALL had a rate constant similar to that of the force (7-10 s(-1)), but that of the sixth ALL was apparently faster. The myosin-induced component of troponin reflection was the only one with negative polarity, and was too slow to be analyzed with this protocol. The results suggest that the three regulation-related proteins change their structures with different rate constants, and the significance of these findings is discussed in the context of a cooperative thin-filament activation mechanism.
Figures

References
-
- Ebashi S., Endo M. Calcium and muscle contraction. Prog. Biophys. Mol. Biol. 1968;18:123–183. - PubMed
-
- Gordon A.M., Homsher E., Regnier M. Regulation of contraction in striated muscle. Physiol. Rev. 2000;80:853–924. - PubMed
-
- Iwamoto H. Physiological consequences of thin filament cooperativity for vertebrate striated muscle contraction: a theoretical study. J. Muscle Res. Cell Motil. 2006;27:21–35. - PubMed
-
- Lehrer S.S., Morris E.P. Dual effects of tropomyosin and troponin-tropomyosin on actomyosin subfragment-1 ATPase. J. Biol. Chem. 1982;257:8073–8080. - PubMed
-
- Bremel R.D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat. New Biol. 1972;238:97–101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

