Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss
- PMID: 19186164
- PMCID: PMC2673052
- DOI: 10.1016/j.neuron.2008.11.024
Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss
Abstract
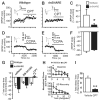
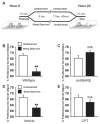
Astrocytes modulate neuronal activity by releasing chemical transmitters via a process termed gliotransmission. The role of this process in the control of behavior is unknown. Since one outcome of SNARE-dependent gliotransmission is the regulation of extracellular adenosine and because adenosine promotes sleep, we genetically inhibited the release of gliotransmitters and asked if astrocytes play an unsuspected role in sleep regulation. Inhibiting gliotransmission attenuated the accumulation of sleep pressure, assessed by measuring the slow wave activity of the EEG during NREM sleep, and prevented cognitive deficits associated with sleep loss. Since the sleep-suppressing effects of the A1 receptor antagonist CPT were prevented following inhibition of gliotransmission and because intracerebroventricular delivery of CPT to wild-type mice mimicked the transgenic phenotype, we conclude that astrocytes modulate the accumulation of sleep pressure and its cognitive consequences through a pathway involving A1 receptors.
Figures

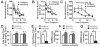
Comment in
-
Glia, adenosine, and sleep.Neuron. 2009 Jan 29;61(2):156-7. doi: 10.1016/j.neuron.2009.01.005. Neuron. 2009. PMID: 19186158 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

