Mapping of class I and class II odorant receptors to glomerular domains by two distinct types of olfactory sensory neurons in the mouse
- PMID: 19186165
- PMCID: PMC3013286
- DOI: 10.1016/j.neuron.2008.11.010
Mapping of class I and class II odorant receptors to glomerular domains by two distinct types of olfactory sensory neurons in the mouse
Abstract
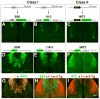
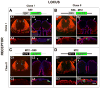
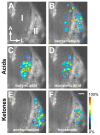
The repertoire of approximately 1200 odorant receptors (ORs) is mapped onto the array of approximately 1800 glomeruli in the mouse olfactory bulb (OB). The spatial organization of this array is influenced by the ORs. Here we show that glomerular mapping to broad domains in the dorsal OB is determined by two types of olfactory sensory neurons (OSNs), which reside in the dorsal olfactory epithelium. The OSN types express either class I or class II OR genes. Axons from the two OSN types segregate already within the olfactory nerve and form distinct domains of glomeruli in the OB. These class-specific anatomical domains correlate with known functional odorant response domains. However, axonal segregation and domain formation are not determined by the class of the expressed OR protein. Thus, the two OSN types are determinants of axonal wiring, operate at a higher level than ORs, and contribute to the functional organization of the glomerular array.
Figures






References
-
- Bozza T, McGann JP, Mombaerts P, Wachowiak M. In vivo imaging of neronal activity by targeted expression of a genetically encoded probe in the mouse. Neuron. 2004;42:9–21. - PubMed
-
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. - PubMed
-
- Buck LB. Information coding in the vertebrate olfactory system. Annu Rev Neurosci. 1996;19:517–544. - PubMed
-
- Bulfone A, Wang F, Hevner R, Anderson S, Cutforth T, Chen S, Meneses J, Pedersen R, Axel R, Rubenstein JL. An olfactory sensory map develops in the absence of normal projection neurons or GABAergic interneurons. Neuron. 1998;21:1273–1282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

